Recent studies have clearly established an inverse relationship between suboptimal antenatal and postnatal environments and the development of adult diseases, including the metabolic syndrome (obesity, atherogenic dyslipidemia, hypertension, insulin resistance); type 2 diabetes; cardiovascular disease; neurologic disorders; and mental illness.1,2,3,4
These observations have been confirmed in multiple human populations1,5 and in numerous animal studies across multiple species4,6,7,8. It is thus clear that the environment of mother, baby and child is a key contributor to diseases and conditions that account for approximately one third of the global burden of disease, affecting both developed and developing countries. Although adverse antenatal and postnatal environments increase the risk of particular adult diseases, not all individuals exposed to these environments develop these conditions suggesting that an individual’s genotype may contribute to the eventual outcome.9,10,11,12 It has therefore been hypothesised that interactions between an individual’s genotype and the environment underlie the developmental origins of health and disease (DOHaD). See Figure 1:
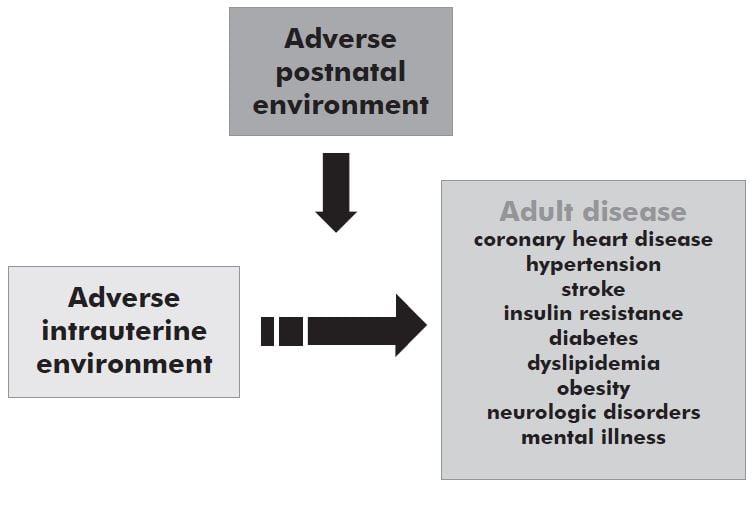
In 1988, retrospective epidemiological studies in British cohorts reported an inverse relationship between birth size and rates of mortality from coronary heart disease across the normal birth weight range.2,3 Subsequently, similar relationships were discovered for other components of the metabolic syndrome, including hypertension, stroke, insulin resistance, type 2 diabetes and dyslipidemia.4 Over the next decade, numerous studies were performed in different populations, both in the developed and developing world, confirming this relationship.1,5 These studies led to the ‘fetal origins’ hypothesis that suggested that events in utero which reduced fetal growth permanently altered the structure and physiology of the offspring such that the risk of heart disease and diabetes in later life was increased.
Animal models have provided the most direct evidence to support the DOHaD thesis.4 Programming has been demonstrated in sheep13, guinea pigs6, pigs, mice and rats7,14 and can be induced by: 1) prenatal under-nutrition (in total caloric or protein dietary content); 2) unbalanced nutrition (for example, maternal high-lard diet); 3) impairing utero-placental perfusion; or 4) maternal exposure to synthetic glucocorticoids.4 Animal studies have demonstrated another important concept, namely, that the fetus need not be manipulated for the relationships between birth size and cardiovascular and metabolic outcomes to be observed.4,15,16 The environmental cue can occur in early or late pregnancy, or within the peri-conceptual period.17 Thus, the term ‘fetal origins’ has now been replaced by ‘developmental origins’.
The most frequent misunderstanding in the DOHaD hypothesis has been the role of birth size. Birth size is simply a crude surrogate reflecting the interactions between the fetal environment and the fetal genome.4 There is a growing body of evidence from both animal7,18-22 and human studies4,23,24 that environmental cues during pregnancy need not alter birth weight to alter long-term outcomes. For example, in the Dutch winter famine, women who ate less than 800 calories a day during the first trimester gave birth to normal-sized infants who later became obese.23,24 Programming is not a process confined to the extremes in fetal growth, but rather one that accompanies the adaptations that every fetus makes to its environment, including subtle variations in growth.4 Indeed, these relationships exist across the normal birth weight range. Whilst initial studies of the DOHaD focused on events that occurred before birth, more detailed analysis of human cohorts25,26,27 and animal studies7 have demonstrated interactions between antenatal and postnatal environment. Those who had the most adverse intrauterine environment and the fastest weight gain after birth were shown to be at greatest risk for programming of the metabolic syndrome. Further, human and animal studies have shown clear differences between males and females, not only in the response to adverse antenatal and postnatal environments, but also in the mechanisms that underlie programming.28,29,30
Although a clear relationship exists between adverse antenatal and postnatal environments and the risk of adult disease, there is growing evidence that genetic polymorphisms modulate this relationship. This is illustrated by the influence of polymorphisms in the peroxisome proliferator-activated receptor (PPAR)-γ2 gene on the relationship between size at birth and adult diseases including: 1) insulin sensitivity and metabolism9,31; 2) hypertension12; 3) obesity11 and 4) dyslipidemia10. The well-known association between small body size at birth and insulin resistance9 and/or hypertesion12 was seen only in individuals with the high-risk Pro12Pro allele. Similarly, polymorphisms in the glucocorticoid receptor (GR), a key control element in the hypothalamic pituitary adrenal axis, have been implicated in determining obesity32,33,34, hypertension35, hypercholesterolemia35 and responses to psychosocial stress in adults33. Thus complex interactions between genes and the environment modulate developmental programming of adult disease.
Over the last 18 months, in conjunction with international collaborators, we have completed the first analyses investigating the association of the fat mass and obesity (FTO) gene with antenatal and postnatal growth trajectories. We have shown that the AA genotype of the SNP rs9939609 in the FTO gene (which has been found to predispose to diabetes through an effect on BMI in adults) was associated with symmetric intrauterine growth restriction beginning as early as 28 weeks gestation. The same polymorphism in FTO was independently associated with increased body mass index (BMI) in males (but not females) at eight, 14 and 17 years of age in multivariate analyses adjusted for birth weight, smoking, socio-economic status, nutrition and activity. Breastfeeding was associated with reduced BMI in childhood/adolescence, in males but not females, independent of FTO genotype. In both sexes, increased weight gain over the first year was associated with higher BMI in adolescence. The obesogenic allele (AA) in FTO was also associated with greater weight gain in the first year compared to non-obesogenic allele (TT). Gene-environment interactions were identified between FTO and measures of nutrition. The impact of weight gain in the first year of life on BMI at eight, 14 and 17 years of age was greater in AA than TT genotypes. Further, the reduction in BMI at 8 and 14 years of age in males associated with breastfeeding was greater in AA than TT genotypes. There was a significant interaction between FTO genotype and diet at 17 years of age in females, with an even greater reduction in BMI seen in AA than TT genotypes in those with a prudent/healthy diet. We have found evidence of gene-environment interactions between childhood nutrition and the FTO gene acting on measures of childhood obesity. These findings highlight the complex pathways underlying the development of obesity and raise the possibility of reducing the prevalence of this condition by targeted interventions in those at greatest genetic risk.
We have also identified a number of SNPs within key candidate genes (leptin, leptin receptor, adiponectin, CRP, IGF-2BP, insulin receptor, mineralocorticoid receptor and others) with associations with antenatal growth trajectories, biometric measures at birth, postnatal growth trajectories, insulin resistance at 14 years of age and non-alcoholic fatty liver disease at 16 years of age. Moreover, we have identified a number of gene-environment interactions between some of these SNPs and measures of nutrition (breastfeeding, healthy/prudent dietary pattern) suggesting the possibility of potential interventions to limit the impact of some of the SNPs associated with increased risk of obesity, insulin resistance and non-alcoholic fatty liver disease.
Epigenetics refers to covalent modification of DNA and core histones that regulate gene activity without altering the nucleotide sequence of DNA. There is growing evidence that the methylation status of genomically imprinted genes can be altered with consequences for subsequent organ growth and function.36,37
Importantly, the epigenetic lability of imprinted genes is not limited to the pre-implantation period and includes the early postnatal period. Recent studies have demonstrated that retrotransposons are elements within the genome that may also be epigenetically labile to early nutrition.38,39 The application of epigenetic approaches and the determination of imprinted or non-imprinted genes and transposon insertion sites that are targets for early nutritional effects on epigenetic gene regulation are important new areas of investigation40 which have great potential to uncover important mechanisms underlying DOHaD.
Identification at birth of genetic signatures that enhance the risk of coronary heart disease, stroke, diabetes, obesity, neurologic disorders or mental illness will provide opportunities to develop lifestyle or medical intervention strategies aimed at preventing these adverse outcomes. The potential to impact on global disease burden and the opportunity to establish healthy life-long trajectories for children is immense.
References
- Barker DJ. Mothers, babies and health in later life. Edinburgh: Churchill Livingstone, 1998.
- Barker DJ, Osmond C. Low birth weight and hypertension. BMJ 1988; 297:134-5.
- Barker DJ, Winter PD, Osmond C, Margetts B, Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet 1989; 2:577-80.
- Gluckman PD, Hanson MA. Developmental origins of disease paradigm: a mechanistic and evolutionary perspective. Pediatr Res. 2004; 56:311-7.
- Osmond C, Barker DJ. Fetal, infant, and childhood growth are predictors of coronary heart disease, diabetes, and hypertension in adult men and women. Environ Health Perspect. 2000; 108 Suppl 3:545-53.
- Matthews SG. Early programming of the hypothalamo-pituitary-adrenal axis. Trends Endocrinol Metab. 2002; 13:373-80.
- Bertram CE, Hanson MA. Animal models and programming of the metabolic syndrome. Br Med Bull. 2001; 60:103-21.
- Newnham JP. Is prenatal glucocorticoid administration another origin of adult disease? Clin Exp Pharmacol Physiol. 2001; 28:957-61.
- Eriksson JG, Lindi V, uusitupa M, et al. The effects of the Pro12Ala polymorphism of the peroxisome proliferator-activated receptor-gamma2 gene on insulin sensitivity and insulin metabolism interact with size at birth. Diabetes 2002; 51:2321-4.
- Eriksson J, Lindi V, uusitupa M, et al. The effects of the Pro12Ala polymorphism of the PPARgamma-2 gene on lipid metabolism interact with body size at birth. Clin Genet. 2003; 64:366-70.
- Pihlajamaki J, Vanhala M, Vanhala P, Laakso M. The Pro12Ala polymorphism of the PPAR gamma 2 gene regulates weight from birth to adulthood. Obes Res. 2004; 12:187-90.
- Yliharsila H, Eriksson JG, Forsen T, et al. Interactions between peroxisome proliferator-activated receptor-gamma 2 gene polymorphisms and size at birth on blood pressure and the use of antihypertensive medication. J Hypertens. 2004; 22:1283-7.
- Moss TJ, Sloboda DM, Gurrin LC, Harding R, Challis JR, Newnham JP. Programming effects in sheep of prenatal growth restriction and glucocorticoid exposure. Am J Physiol Regul Integr Comp Physiol. 2001; 281:R960-70.
- Matthews SG, Owen D, Banjanin S, Andrews MH. Glucocorticoids, hypothalamo-pituitary-adrenal (HPA) development and life after birth. Endocr Res. 2002; 28:709-18.
- Kind KL, Simonetta G, Clifton PM, Robinson JS, Owens JA. Effect of maternal feed restriction on blood pressure in the adult guinea pig. Exp Physiol. 2002; 87:469-77.
- Kind KL, Clifton PM, Grant PA, et al. Effect of maternal feed restriction during pregnancy on glucose tolerance in the adult guinea pig. Am J Physiol Regul Integr Comp Physiol. 2003; 284:R140-52.
- Kwong WY, Wild AE, Roberts P, Willis AC, Fleming TP. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000; 127:4195-202.
- Oliver MH, Breier BH, Gluckman PD, Harding JE. Birth weight rather than maternal nutrition influences glucose tolerance, blood pressure, and IGF-I levels in sheep. Pediatr Res. 2002; 52:516-24.
- Hanson M. Birth weight and the fetal origins of adult disease. Pediatr Res. 2002; 52:473-4.
- Bertram CE, Hanson MA. Prenatal programming of postnatal endocrine responses by glucocorticoids. Reproduction 2002; 124:459-67.
- Hoet JJ, Hanson MA. Intrauterine nutrition: its importance during critical periods for cardiovascular and endocrine development. J Physiol. 1999; 514 ( Pt 3):617-27.
- Lingas RI, Matthews SG. A short period of maternal nutrient restriction in late gestation modifies pituitary-adrenal function in adult guinea pig offspring. Neuroendocrinology 2001; 73:302-11.
- Ravelli GP, Stein ZA, Susser MW. Obesity in young men after famine exposure in utero and early infancy. N Engl J Med. 1976;295:349-53.
- Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811-6.
- Eriksson JG, Forsen T, Tuomilehto J, Winter PD, Osmond C, Barker DJ. Catch-up growth in childhood and death from coronary heart disease: longitudinal study. BMJ 1999;318:427-31.
- Burke V, Beilin LJ, Simmer K, et al. Predictors of body mass index and associations with cardiovascular risk factors in Australian children: a prospective cohort study. Int J Obes Relat Metab Disord. 2004.
- Burke V, Beilin LJ, Blake KV, et al. Indicators of fetal growth do not independently predict blood pressure in 8-year-old Australians: a prospective cohort study. Hypertension 2004;43:208-13.
- McCormick CM, Smythe JW, Sharma S, Meaney MJ. Sex-specific effects of prenatal stress on hypothalamic-pituitary-adrenal responses to stress and brain glucocorticoid receptor density in adult rats. Brain Res Dev Brain Res. 1995;84:55-61.
- Dean F, Matthews SG. Maternal dexamethasone treatment in late gestation alters glucocorticoid and mineralocorticoid receptor mRNA in the fetal guinea pig brain. Brain Res. 1999;846:253-9.
- Andrews MH, Matthews SG. Programming of the hypothalamo-pituitary-adrenal axis: serotonergic involvement. Stress 2004;7:15-27.
- Laakso M. Gene variants, insulin resistance, and dyslipidaemia. Curr Opin Lipidol. 2004;15:115-20.
- van Rossum EF, Voorhoeve PG, te Velde SJ, et al. The ER22/23EK polymorphism in the glucocorticoid receptor gene is associated with a beneficial body composition and muscle strength in young adults. J Clin Endocrinol Metab. 2004;89:4004-9.
- Wust S, Van Rossum EF, Federenko IS, Koper JW, Kumsta R, Hellhammer DH. Common polymorphisms in the glucocorticoid receptor gene are associated with adrenocortical responses to psychosocial stress. J Clin Endocrinol Metab. 2004;89:565-73.
- Rosmond R. Association studies of genetic polymorphisms in central obesity: a critical review. Int J Obes Relat Metab Disord. 2003; 27:1141-51.
- Di Blasio AM, van Rossum EF, Maestrini S, et al. The relation between two polymorphisms in the glucocorticoid receptor gene and body mass index, blood pressure and cholesterol in obese patients. Clin
Endocrinol. (Oxf) 2003;59:68-74. - Young LE, Fernandes K, McEvoy TG, et al. Epigenetic change in IGF2R is associated with fetal overgrowth after sheep embryo culture. Nat Genet. 2001;27:153-4.
- Waterland RA, Jirtle RL. Early nutrition, epigenetic changes at transposons and imprinted genes, and enhanced susceptibility to adult chronic diseases. Nutrition 2004;20:63-8.
- Wolff GL, Kodell RL, Moore SR, Cooney CA. Maternal epigenetics and methyl supplements affect agouti gene expression in Avy/a mice. Faseb J. 1998;12:949-57.
- Waterland RA, Jirtle RL. Transposable elements: targets for early nutritional effects on epigenetic gene regulation. Mol Cell Biol. 2003; 23:5293-300.
- McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005; 85:571-633.






Leave a Reply