Although fortunately uncommon, abnormal placentation can result in massive haemorrhage at the time of delivery, with blood loss often reported of two to five litres and in some cases up to 20 litres.1 Even in cases of planned caesarean hysterectomy, haemostasis can be difficult to obtain.
This occurs most notably with placenta percreta, when placental tissue has invaded beyond the uterus into surrounding structures, most commonly the bladder. Such complications of pregnancy are a well-recognised cause of maternal death.
An increasing number of centres now include interventional radiology (IR) as part of the delivery protocol when abnormal placentation is known or suspected, with the aim of reducing peripartum blood loss. The data supporting the use of IR mostly comes from case reports and small retrospective cohort studies.2-6 While no single IR protocol has been demonstrated as the method of choice, there are a number of key principles that are important to ensure that the best use is made of IR in the peripartum period.
Vascular anatomy in abnormal placentation
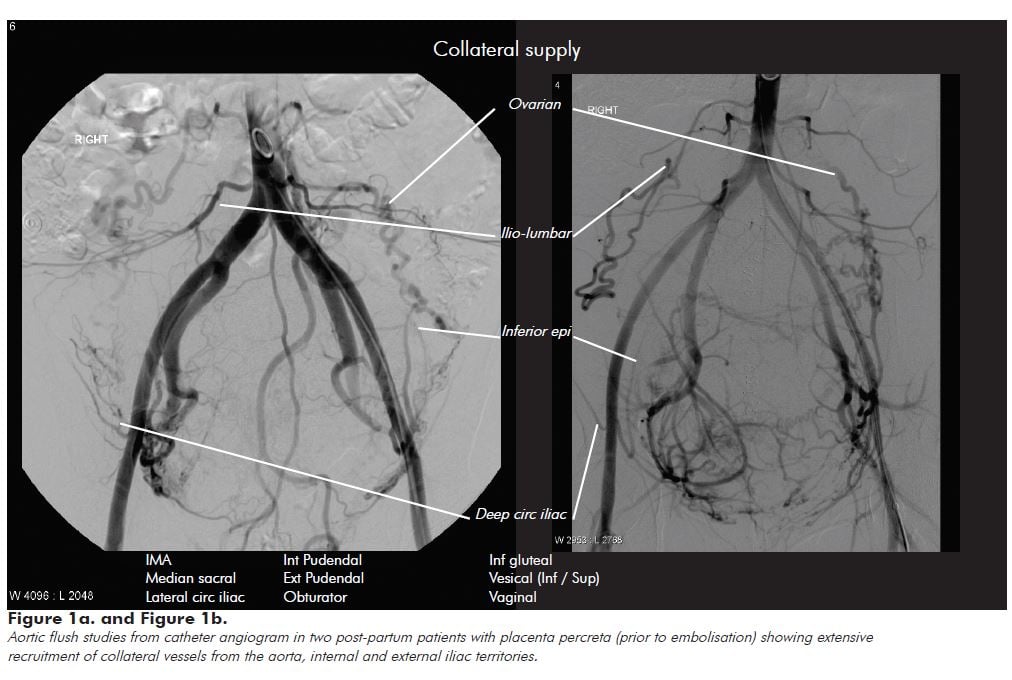
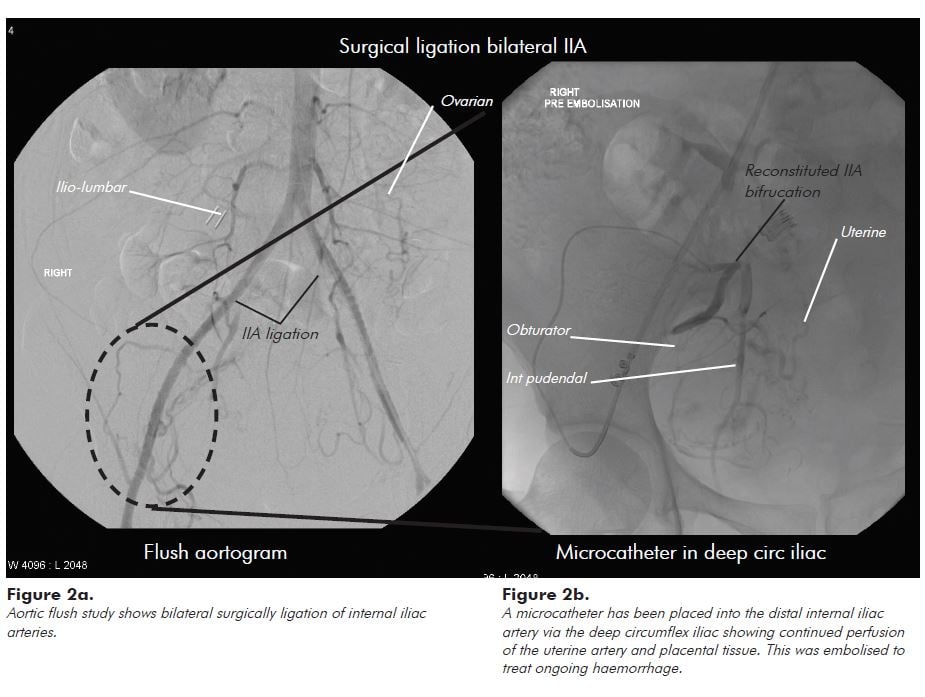
Interventional radiology can help gain an understanding of the vascular anatomy in such cases, and why haemostasis can be so challenging in these patients. Figure 1 shows the vascular territories recruited in a patient with abnormal placentation. While internal iliac artery branch vessels (the uterine, internal pudendal and vesical) are often the dominant supply vessels, the uterus and placenta obtain extensive collateral supply from aortic (ovarian and ilio-lumbar) and external iliac (lateral circumflex iliac, inferior epigastric, ilio-lumbar, obturator and external pudendal) vascular territories. Figure 2 shows that even after emergency bilateral ligation of the internal iliac arteries for catastrophic haemorrhage, the placenta still derives significant arterial supply from aortic and external iliac artery collaterals.
Postpartum embolisation
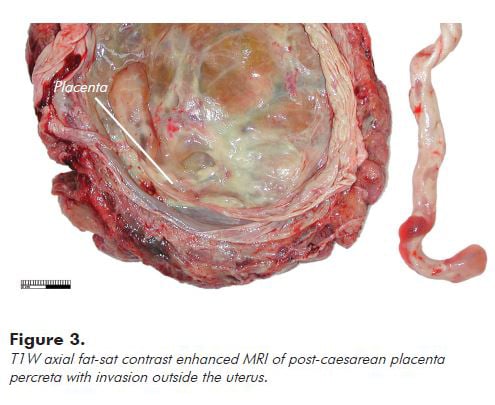
Embolisation of the uterine arterial supply is the main IR treatment option for post-partum haemorrhage (PPH). First reported in 19797, subsequent studies4,8-14 have supported its use in patients haemodynamically stable enough to be transferred to IR. This technique has been shown to have good efficacy when compared to uterine compression sutures, iliac artery ligation or uterine devascularisation. In patients with placenta percreta, embolisation of collateral supply vessels may be necessary to ensure effective treatment. It is important to be aware that placental tissue that invades beyond the uterus, as shown in Figure 3, can be difficult to differentiate from the vascular supply to adjacent pelvic organs, including bladder and bowel, and requires an experienced interventional radiologist to avoid non-target embolisation.15
The guiding principle for patients with abnormal placentation should be to provide permanent distal occlusion of placental arterial supply beyond the main collateral supply arteries. This is best performed using particle embolisation similar to uterine artery embolisation for fibroids. In principle, distal embolisation will reduce the risk of ongoing bleeding from collateral supply that can occur with more proximal embolisation with coils. In patients with haemodynamic instability from ongoing bleeding, many centres will perform embolisation of the internal iliac artery with Gelfoam. This can be effective, but there remains a risk of ongoing bleeding from any collateral supply. Gelfoam embolisation is performed with the expectation of temporary vessel occlusion for three to six weeks, but it can result in permanent vessel occlusion, which is important to remember in this patient population. A decision to either ligate the internal iliac artery intra-operatively, or to embolise the internal iliac artery proximal to bleeding placental tissue, can make subsequent intervention for ongoing bleeding more complex. While we have found it feasible to embolise placental tissue for ongoing bleeding following intra-operative internal iliac artery ligation (Figure 2b), it requires the use of micro-catheters and a thorough understanding of aorto-iliac vascular anatomy.
Balloon occlusion for abnormal placentation
Pre-operative placement of occlusion balloons, first described for the aorta in 199516 and subsequently for the internal iliac arteries14 was introduced with the goal of temporarily reducing uterine and placental blood flow, without the need for permanent iliac artery ligation. Since then, a small number of studies and case reports have been published.1-5,16,17,18 However, probably because of the large number of factors contributing to individual patient blood loss, an unequivocal benefit with use of occlusions balloons has not been demonstrated.
Arterial sheath and balloon placement and management
In the absence of clear evidence supporting the use of occlusion balloons, there is a greater imperative to avoid complications. This requires well documented protocols that should cover the following.
Arterial sheath placement
Many centres will arrange for balloons to be placed by the interventional radiologist in the medical imaging department, with bilateral common femoral artery vascular sheaths. Following sheath placement, patient movement should be kept to a minimum, specifically hip flexion and patient rotation, to avoid displacement of the sheaths or balloons. If an epidural is planned this should be placed prior to transfer for placement of the balloons. If ureteric stent placement is required this can be more challenging to do prior to occlusion balloon placement, but it is preferable to balloon displacement. If ureteric stents are placed in the operating room with arterial sheaths in situ, then full lithotomy positioning should be avoided and the minimum amount of hip flexion possible used.
Balloon placement and inflation
The majority of interventional radiologists will use compliant balloons, which should reduce the risk of vessel injury (dissection or rupture). Non-compliant or standard angioplasty balloons are occasionally used, but can be difficult to match to the vessel lumen and, when mis-matched, can result in either poor vessel occlusion or even dissection.
With careful technique, placement of occlusion balloons in the angiography suite should result in minimal screening and radiation dose. Balloon inflation is most frequently performed following cutting of the umbilical cord, although some obstetricians will only request balloon inflation if haemostasis appears inadequate. Balloons should be removed on completion of the operation to reduce the risk of arterial thromboembolism. If embolisation is necessary because of ongoing bleeding, this can be done leaving the balloon catheters in situ, with the balloons deflated.
Post-operative management
The setting for postoperative care can vary greatly, from a general obstetric ward to an intensive care environment, depending on the patient’s post-operative status. It is important to have an agreed protocol regarding when, and by whom, arterial sheaths are to be removed. This protocol should be easily available in the patient’s notes. Sheaths left in situ for any length of time without proper care can result in significant complications and morbidity7,15 resulting from arterial embolism. The practice in my unit is to remove all sheaths within two hours post-procedure unless postoperative embolisation is planned.
Multi-disciplinary management
Despite the trend of an increasing number of patients presenting with abnormal placentation19, most centres deal with a limited number of intermittent cases and it can be difficult to have a designated group of specialists available for each case. This is especially true because of the significant number of specialist teams that can be involved in addition to obstetrics, including gynae-oncology, anaesthesia, vascular surgery, urology, intensive care and interventional radiology. Nevertheless, it is important to identify a group of clinicians who have an interest in the management of abnormal placentation to allow development of a consistent approach to treatment and familiarity of team members. This facilitates the complex logistical planning when interventional radiology is required. Our hospital requires at least one multi-disciplinary team meeting, with all clinicians likely to be involved in the delivery present, to outline the management plan prior to the procedure.
Interventional radiology can provide important adjunctive support to this complex patient group, and specifically those patients at risk of significant or ongoing haemorrhage. Arterial embolisation has been used for over 30 years and appears to have good efficacy. Use of occlusion balloons is reasonable to consider, but requires coordinated multi-disciplinary management and well-documented, easily accessible protocols to ensure risks are minimised. Familiarity of all team members is critically important when dealing with a rapidly changing clinical situation, and this is greatly facilitated by a pre-operative multi-disciplinary meeting.
Talat Uppal (FRANZCOG)
‘Sisters by chance….friends by choice’ was engraved on the photo frame I bought for my sister during her time in hospital. A young woman, her first child had been delivered by emergency caesarean section, after making it to 8 cm dilatation. Her new pregnancy was precious indeed, the result of IVF treatment, following years of secondary infertility.
My sister was known to have placenta praevia, but from 34 weeks features of percreta had been noted on ultrasound, following a small antepartum haemorrhage. An MRI performed at 36 weeks then raised the possibility of invasion into the bladder.
When I heard the words, ‘Your sister has had a four-litre blood loss…and has had a hysterectomy…there is currently an element of DIC….but she will go home with her son,’ I remember thinking how challenging it was being on the other side of the fence; no longer the obstetrician, but the relative of a patient. I was hearing about my sister battling for life in the intensive care unit: my only sibling, herself an intensive care registrar. Waves of relief flooded through me that my mother had not understood the meaning of ‘DIC’….and hence could be spared knowledge of the gravity of the situation.
I remember sobbing as I held my sister’s swollen hand amid the clutter of monitors and leads. I could not believe the reality of what was happening, my sister so passionate about her own ICU work. I was shocked to see her nasogastric tube suctioned of fresh blood. Thank goodness so much preparation was done: anaesthetic and haematology support well organised. What if it had been an emergency case at 2 o’clock in the morning? Fortunately, the entire healthcare team caring for us was fabulous and we were really well supported during this period.
There is an almost unfathomable finality about losing one’s uterus. It can leave a patient feeling incomplete. However, I have had so many experiences with my sister and her children since that terrible time. She is fit and healthy and able to look after her two children. It has felt like the beginning of a new journey for us.
References
- Weeks SM, Stroud TH, Sandhu J, Mauro MA, Jaques PF. Temporary balloon occlusion of the internal iliac arteries for control of hemorrhage during caesarean hysterectomy in a patient with placenta previa and placenta increta. Journal of Vascular and Interventional Radiology 2000; 11:622-624.
- Tan CH, Tay KH, Sheah K, et al. Perioperative endovascular internal iliac artery occlusion balloon placement in management of placenta accreta. AJR Am J Roentgenol. 2007; 189:1158-1163.
- Shrivastava V, Nageotte M, Major C, Haydon M, Wing D. Case-control comparison of caesarean hysterectomy with and without prophylactic placement of intravascular balloon catheters for placenta accreta. Am J Obstet Gynecol. 2007; 197:402 e401-405.
- Ojala K, Perala J, Kariniemi J, Ranta P, Raudaskoski T, Tekay A. Arterial embolization and prophylactic catheterization for the treatment for severe obstetric hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 2005 Nov; 84:1075-1080.
- Levine AB, Kuhlman K, Bonn J. Placenta accreta: comparison of cases managed with and without pelvic artery balloon catheters. J Matern Fetal Med. 1999; 8:173-176.
- Chou MM, Hwang JI, Tseng JJ, Ho ES. Internal iliac artery embolization before hysterectomy for placenta accreta. Journal of Vascular and Interventional Radiology 2003 Sept; 14:1195-1199.
- Brown BJ, Heaston DK, Poulson AM, Gabert HA, Mineau DE, Miller FJ. Uncontrollable postpartum bleeding: a new approach to haemostasis through angiographic arterial embolization. Obstet Gynecol. 1979; 54:361-365.
- Doumouchtsis SK, Papageorghiou AT, Arulkumaran S. Systematic review of conservative management of postpartum haemorrhage: what to do when medical treatment fails. Obstet Gynecol Surv. 2007; 62:540-547.
- Vedantham S, Goodwin S, McLucas B, Mohr G. Uterine artery embolization: An underused method of controlling pelvic hemorrhage. American Journal of Obstetrics and Gynecology 1997 Apr; 176:938-948.
- Pelage J-P, Le Dref O, Jacob D, Soyer P, Herbreteau D, Rymer R. Selective arterial embolization of the uterine arteries in the management of intractable post-partum hemorrhage. Acta Obstetricia et Gynecologica Scandinavica 1999 Sep; 78:698-703.
- Hong TM, Tseng HS, Lee RC, Wang JH, Chang CY. Uterine artery embolization: an effective treatment for intractable obstetric haemorrhage. Clinical Radiology 2004; 59:96 101.
- Hansch E, Chitkara U, McAlpine J, El-Sayed Y, Dake M, Razavi M. Pelvic arterial embolization for control of obstetric haemorrhage: A five-year experience. American Journal of Obstetrics and Gynecology 1999 Jun; 180:1454-1460.
- Dinkel HP, Durig P, Schnatterbeck P, Triller J. Percutaneous treatment of placenta percreta using coil embolization. Journal of Endovascular Therapy: Official Journal of the International Society of Endovascular Specialists 2003; 10:158-162.
- Alkazaleh F, Geary M, Kingdom J, Kachura JR, Windrim R. Elective non-removal of the placenta and prophylactic uterine artery embolization postpartum as a diagnostic imaging approach for the management of placenta percreta: a case report. Journal of Obstetrics and Gynaecology Canada 2004; 26:743-746.
- Porcu G, Roger V, Jacquier A, et al. Uterus and bladder necrosis after uterine artery embolisation for postpartum haemorrhage. BJOG 2005; 112:122-123.
- Paull JD, Smith J, Williams L, Davison G, Devine T, Holt M. Balloon occlusion of the abdominal aorta during caesarean hysterectomy for placenta percreta. Anaesthesia and Intensive Care 1995; 23:731-734.
- Shih J, Liu K, Shyu M. Temporary balloon occlusion of the common iliac artery: New approach to bleeding control during cesarean hysterectomy for placenta percreta. American Journal of Obstetrics and Gynecology 2005 Nov; 193:1756-1758.
- Dubois J, Garel L, Grignon A, Lemay M, Leduc L. Placenta percreta: balloon occlusion and embolization of the internal iliac arteries to reduce intraoperative blood losses. American Journal of Obstetrics and Gynecology 1997; 176:723-726.
- Armstrong CA, Harding S, Matthews T, Dickinson JE. Is placenta accreta catching up with us? Aust N Z J Obstet Gynaecol. 2004; 44:210-213.





Leave a Reply