This practical guide to examination of the placenta is principally aimed at those practitioners who do not have immediate access to a laboratory that is able to deal with placentas on the day of delivery.
This guide includes an approach to examination of placentas from both liveborn and stillborn infants and is relevant to both the second and third trimesters. The Perinatal Society of Australia and New Zealand (PSANZ) guidelines for the investigation of perinatal death cover placental examination in detail.1
In a study of stillbirths at 23 to 40 weeks gestation, 12 per cent of causes of intrauterine fetal death (IUFD) were found in the fetus, and 88 per cent of causes were found in the placenta, cord and membranes.2 There is a large body of literature that addresses both the importance of the placental contribution to poor pregnancy outcomes and also the poor rate and quality of placental examination. In one survey, the authors found that the hospital staff examined only one-third of the placentas that were recommended, using the College of American Pathologists practice guidelines.3
The optimum handling of the placenta following delivery is to send the fresh placenta and cord, with a detailed request form, to the laboratory. The pathologist performs macroscopic and microscopic examination, takes swabs for microbiology, sampling for genetics (if appropriate) and determines chorionicity in multiple pregnancies.4 The pathology report that the lead maternity carer (LMC) receives, commonly reflects the quality of the clinical information provided on the request form. Most pathologists appreciate that many of the placentas that are sent for examination are delivered in the setting of clinical urgency regarding either the mother or baby, or both. This may, in part, explain the paucity of information on many request forms – the barest information being ‘placenta’. In response, some laboratories have tailored a specific placental request form that has largely overcome this deficiency.
Many of the antenatally recognised complicated pregnancies are electively delivered in tertiary centres. Some of these centres have a perinatal pathology service and the clinicians may expect a gold-standard placental examination. Not all babies have read the ‘birth plan’ and many women with complicated pregnancies may labour precipitately or unexpectedly in centres remote from the anticipated site of delivery. Some babies are born unexpectedly ‘flat’ at term. When these and other adverse events happen, the immediate postpartum attention is with the mother and baby. However, it is important to recall that the placenta may hold the answer to the frequently asked questions: why did it happen and will it happen again?
Despatch to a pathologist
Placental examination guidelines recommend that the placenta and cord are sent fresh to the laboratory. If the site of delivery is remote from your nearest laboratory there are several options:
a. Speak to the laboratory/pathologist and ask for advice re: samples and method of packaging and despatch.
b. The placenta can be placed in refrigerator and despatched the following day(s), if necessary. Do not place the placenta in saline. (This may confuse the laboratory staff into thinking the fluid is formalin and thus the specimen does not have to be examined immediately. Saline is not a fixative). The placenta will stay moist if placed in a sealed plastic bag. Do not freeze – this produces extensive artefacts.
c. If the above are not possible/practical, take microbiology swabs as per guidelines and sample for genetics and put placenta in formalin solution and despatch.
d. If the placenta is to be returned to the parents after examination, this should be included in the despatched paperwork.
e. In the case of a stillbirth that is for autopsy, the fresh placenta should accompany the baby to the mortuary.
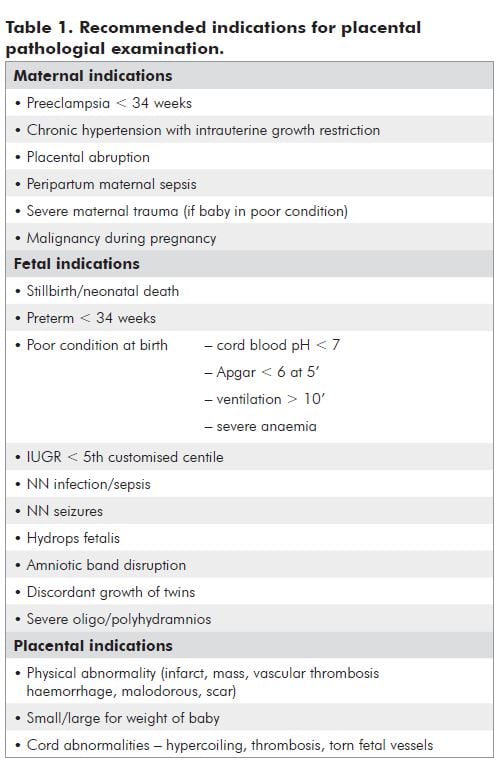
There are a number of observations that the doctor or midwife can make at the time of delivery that may improve information gained. There may be findings that prompt a maternal or fetal investigation. After delivery, document the following:
- Placental weight
- Liquor details
- Cord details
- Maternal and fetal surfaces
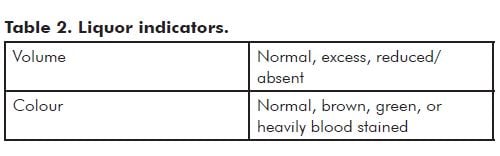
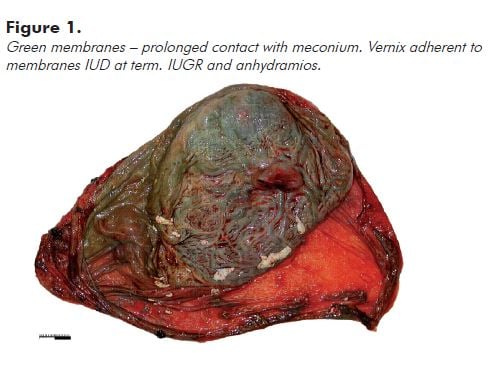
Note: Prolonged contact with meconium usually leads to opaque olive green discolouration of the membranes and cord (see Figure 1). Fresh meconium shortly before delivery usually does not stain the membranes significantly.
Heavily blood-stained liquor and a pale floppy baby may be the result of torn fetal vessels – this may occur in utero or intrapartum. Marginal and velamentous cord insertions are particularly vulnerable to trauma. If you are too remote to expect to send the placenta to a pathologist fresh, after you have done swabs and genetic samples (if indicated), gently wash the insertion site and examine the vessels. A significant traumatic defect in a vessel is usually not subtle.
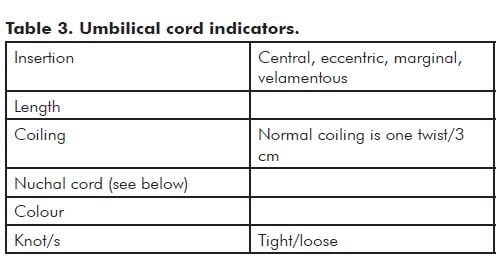
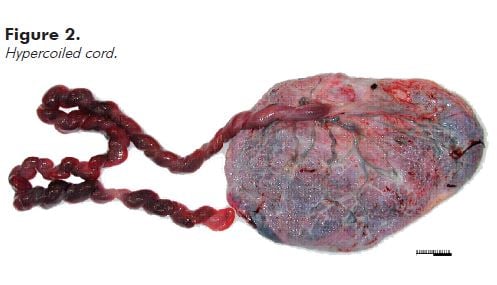
Note: Hypercoiling (also known as torsion) and undercoiling of the cord are associated with adverse pregnancy outcomes.5 As a general rule, if the cord looks like a coiled telephone cord, it is hypercoiled (see Figure 2).
The nuchal cord is usually only seen by the people performing the delivery. Nuchal cords are common and most are of no significance.
Record the number of loops about the neck or other parts of the body. Also record if there was evidence of skin compression – either blanching or an obvious indentation on any part of the body. Was the cord looped over the shoulder and was it necessary to cut the cord to facilitate delivery? In these cicumstances, the length of the cord and coiling index should be recorded with this information.
In the event of a fetal death or poor condition at birth, this description may be the record that the pathologist, neonatologist or LMC returns to, to explain the poor outcome.
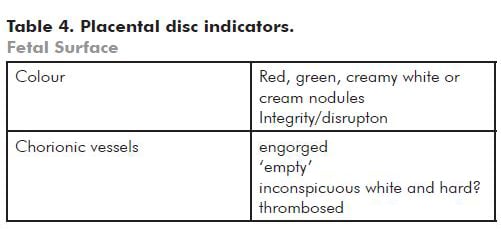
Note: Red discolouration is usually related to fresh blood staining. Green indicates meconium staining – if there is a bowel atresia it may be caused by fetal vomiting. Cream/yellow is usually due to choriomanionitis and the amnion may be friable.
Small white nodules of amnion nodosum may be seen on the amnion surface and are usually seen in association with severe prolonged oligohydramnios.
If there has been disruption of the amnion, there may appear to be short or long strands attached to the fetal surface – these are tough and different to normal amnion. he baby may show features of amniotic bands. If the fetus is stillborn, examine the umbilical cord for a constricting amniotic band.
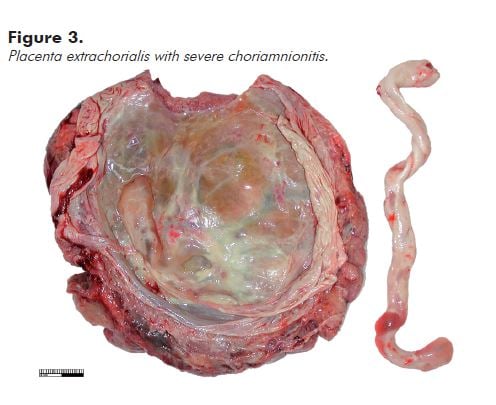
A circumferential, slightly raised white/cream rim just inside the disc perimeter (placental extrachorialis) represents an historic organised marginal haemorrhage (see Figure 3). This is commonly complicated by premature rupture of the membranes.
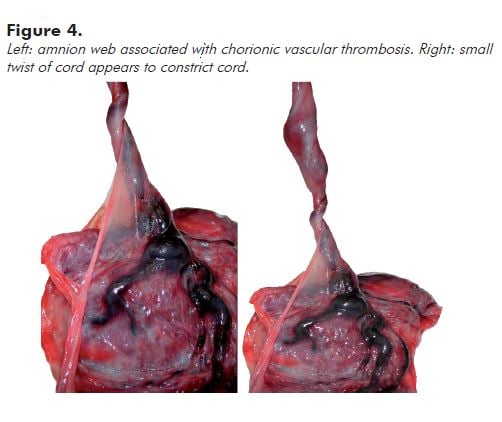
Note: Very engorged vessels may reflect obstruction to flow in the umbilical cord. Look to see if there is an amnion web inserted along the cord at a variable distance from the fetal surface. This anchors the cord and may cause (intermittent) occlusion associated with fetal movement. You can demonstrate a potential for obstruction by twisting the cord 90 degrees or less (see Figure 4).
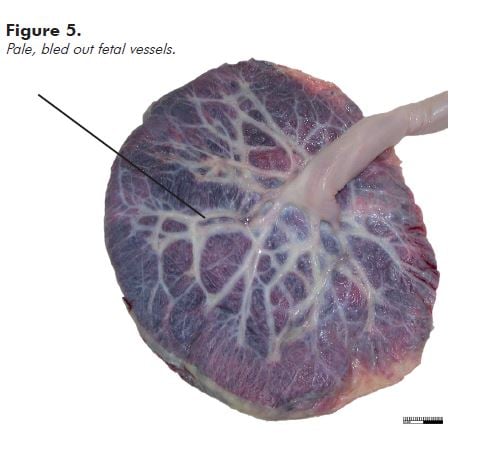
‘Empty’ fetal vessels (see Figure 5) (without significant hydrops) are seen most commonly in associated with acute exsanguination, as in a large fetomaternal haemorrhage; haemorrhage from a fetal vessel as already described; or haemorrhage in the fetus, most usually an intracranial haemorrhage. Do a Kleihauer-Betke test. Tell the laboratory that you are waiting for the result. If the result indicates that the fetus has bled at least 30 per cent of the blood volume into the maternal circulation, that is the most likely cause of the poor condition or stillbirth.
References
- PSANZ Clinical Practice Guideline for Perinatal Mortality Audit: 2nd edition version 2.2 April 2009. Section 4.2.2 p.76.
- D.Kidron, J.Bernheim, R.Aviram. Placental findings contributing to fetal death, as study of 120 stillbirths between 23 and 40 weeks gestation. Placenta 2009;30:700-704.
- M.K.Spencer, T.Y.Khong. Conformity to guidelines for pathologic examination of the placenta. Rates of submission and listing of clinical indications. Arch Path Lab Med 127 ( 205-207) 2003.
- T.Y.Khong. From delivery suite to laboratory: optimizing returns from placental examination in medico-legal defence. Aust. NZ J Obstet Gynaecol 1997; 37:1:1.
- MWM de Laat et al. Umbilical coiling index in normal and complicated pregnancies. Obstet Gynaecol 2006;107:1049-55.






Leave a Reply