Q&a attempts to provide balanced answers to those curly-yet-common questions in obstetrics and gynaecology for the broader O&G Magazine readership, including Diplomates, Trainees, medical students and other health professionals.
Q
The use of antenatal and postnatal RhD immunoglobulin prophylaxis has successfully reduced the incidence of maternal alloimmunisation to the RhD antigen to less than 1.5 per cent. However, since assessment of an adequate dose of RhDIg is critical to the success of these programs, should fetal maternal haemorrhage screening be widely practised?
a Fetal maternal haemorrhage (FMH) screening is the recommended standard of care post-delivery in RhD negative women delivering an RhD positive baby. The test is undertaken to ensure that the correct dose of RhD immunoglobulin (RhDIg) is administered following delivery. The standard post-delivery dose is 625 IU, sufficient to protect against 6.25ml of fetal red cells.
However, in up to three per cent of deliveries the FMH will be greater, hence the importance of the routine use of this screening test as recommended by the RANZCOG College Statement on Anti-D1 administration in obstetrics.
This case described below highlights the importance of compliance with the national standard of testing for FMH immediately post-delivery, or indeed at any other stage of pregnancy.
Case report
AG, a 29-year-old woman, delivered an apparently non-anaemic RhD positive baby by lower segment caesarean section two days before a request for a crossmatch of two units of red cells for postpartum anaemia. Her blood group was already known to be O RhD negative and she had already received the standard 625 IU RhDIg dose immediately after delivery.
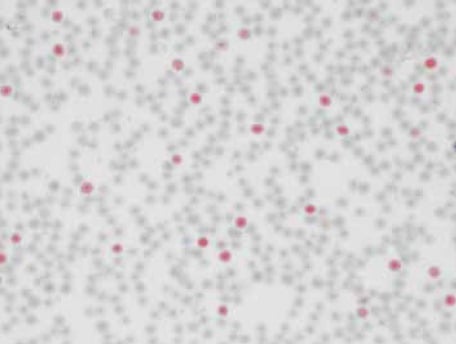
Kleihauer-Betke film showing prominent numbers of RhD positive maternal cells.
The testing at the laboratory performing the crossmatch request (on a sample collected two days after delivery) gave a weak result in the RhD group, which was an unexpected finding. This was confirmed by further testing. Consideration of this result and the fact that the maternal antibody screen failed to detect any trace of the RhDIg given two days earlier led the laboratory to recommend that an FMH screen be performed urgently, given that the 72 hour anti-D administration window was quickly closing. The cord Hb was reported as 118g/l (normal range 136–196g/l), which could be considered as a further marker to suspect a large FMH.
The FMH screen was performed using both Kleihauer-Betke and flow cytometric techniques and returned a result of more than three per cent fetal red cells.
Using the recommended formula it was estimated that an FMH of >70ml had occurred. For haemorrhages greater than 6ml, the recommended dose is 100 IU per ml RhD positive red blood cells. Where large volumes of RhD-Ig need to be administered or the patient has a specific contraindication to intramuscular injections, an intravenous (IV) RhD-Ig preparation should be considered. In discussion with her treating physician a further dose of 13 vials of WinRho RhDIg was recommended to be given intravenously.
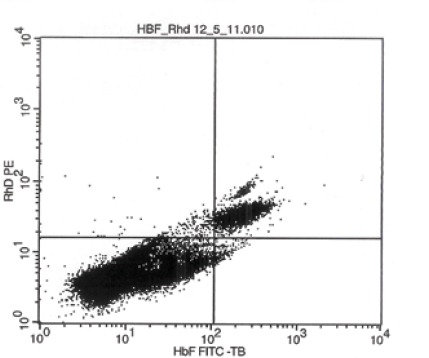
Flow cytometry scan of results showing upper right-hand quadrant HbF / RhD positive cells = 3.19 per cent.






Leave a Reply