More than 300 000 robotic surgical procedures were performed worldwide in 2011. What role is there for robotic surgery in gynaecological oncology in our region?
Urologists have been the early adopters of robotic surgery, with more than 90 per cent of prostatectomies in the USA now being performed robotically. Despite this head start, gynaecology has caught up and, in the USA at least, more robotic gynaecology procedures are now performed than robotic urology procedures. Australia has been slower to adopt the technology and currently there are only three gynaecologists (two of them gynaecological oncology subspecialists) accredited by Intuitive Surgical® as robotic surgeons, although several are currently proceeding along the accreditation pathway.
The initial research and development for robotic surgery was undertaken by a collaboration from Stanford University, NASA and the US military, who envisioned a system by which emergency battlefield surgery could be undertaken by robots located close to the action during wartime, while the surgeons who controlled the robots were able to remain safely behind the frontline. Ultimately, the military recognised this as a flawed concept and sold it to a private consortium (Intuitive Surgical) that has continued to develop and refine the system to what we today know as the daVinci® Surgical System. Although there have been a number of, essentially, false starts by other companies with prototypes, the daVinci Surgical System is currently the only commercially available robotic surgical system. It seems unlikely that any comparable robotic systems will appear in the short term.
The daVinci Surgical System consists of an ergonomically designed surgeon’s console, a patient cart with four interactive robotic arms, a high-performance vision system and patented EndoWrist® instruments.1 The patient cart (the robot itself) has four arms that are connected to the telescope and surgical instruments, and access the body cavity via laparoscopic ports. The console at which the surgeon sits, remote from the patient, includes the ‘masters’ (or hand controls for the robotic instruments); foot pedals that control some of the functions; and a viewing box in which the surgeon has a three-dimensional view of the operative field. Lastly, the system includes a tower that houses the insufflator, light, energy sources and other electronic components. The surgical assistant, nursing staff, anaesthetist and others can view the surgery on standard laparoscopic monitors, but unlike the surgeon they do not see the surgery in three dimensions.
The robot is nothing more than a tool to facilitate laparoscopic surgery, albeit a very sophisticated tool. As such, it conveys many of the established benefits of laparoscopic surgery over laparotomy, including smaller incisions, better cosmesis, less pain, quicker recovery, shorter hospital stay and fewer complications.2 In addition, the robot provides the surgeon with a number of features far superior to its standard laparoscopic counterpart. These include better vision, better instrumentation and better ergonomics. The robotic telescope has two telescopes in its shaft and two cameras, thus projecting a true three-dimensional image for the surgeon’s view. The surgeon’s experience of looking into the viewing box is thus of a high-resolution, magnified, three-dimensional view of the surgical field that encompasses the surgeon’s entire visual field. Compare the robotic surgeon, to whom the operative field comprises his entire visual field and gives the surgeon the sensation of being immersed within the pelvis, to the laparoscopic surgeon who views a small, two-dimensional screen a couple of metres across the operating table that comprises a small part of the visual field. In standard laparoscopic surgery, the greater part of the surgeon’s visual field is filled with all of the potential distractions elsewhere in the operating theatre that must be filtered out.
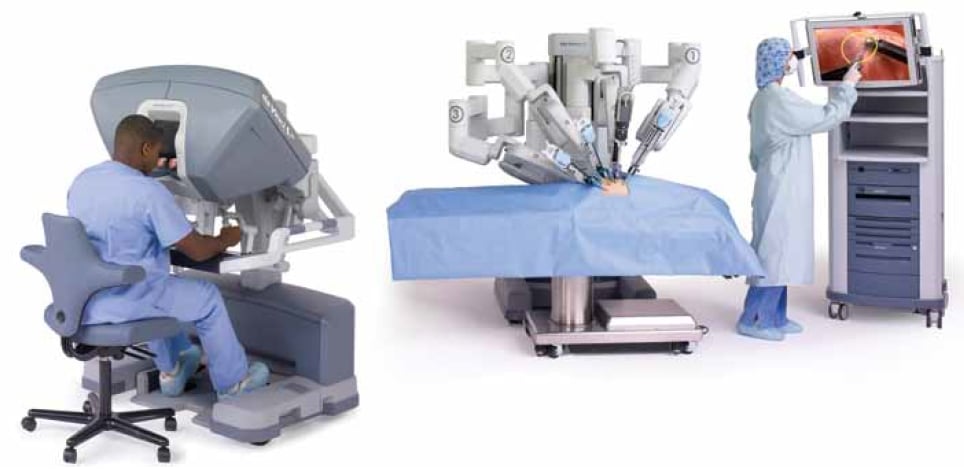
The daVinci Surgical System consists of a surgeon’s console, a patient cart with four interactive robotic arms, a vision system and EndoWrist instruments.
Because laparoscopic surgery uses the abdominal wall as a fulcrum, it requires counterintuitive movement by the surgeon. This means that laparoscopic surgeons must teach their hands to do the opposite movement to that which the brain is indicating they should. This requirement for counterintuitive motion is eliminated by the software in the robotic system (as is any surgeon tremor), so that the robot instruments mimic exactly the surgeon’s hand movements. Further, the robotic instruments are ’wristed‘ and thus can perform movements that mimic the human hand, but at a much finer scale. The EndoWrist instruments have seven degrees of freedom; one more than the human hand. Compare this with the simple open-and-close movement allowed by conventional ’straight sticks‘ laparoscopy. Finally, the robotic surgeon sits comfortably at a console and operates without the need to lift, stretch or strain. This ergonomic improvement results in a much lower potential for surgeon fatigue or injury compared to laparoscopy.
The advantages afforded by the robot will apply to most cases, however, simple procedures may still be undertaken very satisfactorily using conventional laparoscopic equipment and do not justify the extra set up times and expense of robotic surgery. The benefit of the robot will become increasingly apparent as the degree of difficulty of the surgery increases, especially in cases involving morbid obesity, significant pelvic adhesions, large fibroids, severe endometriosis or staging of malignancy requiring pelvic or para-aortic lymph node dissection. Anyone who has performed laparoscopic surgery on morbidly obese patients and struggled against the weight of the abdominal wall to achieve each movement of the handheld instruments would envy the relaxed demeanour of the robotic surgeon, who requires no more physical effort to operate on a 150kg patient as on a 50kg patient. Further, the robotic instruments are able to hinge at the ’wrist‘ and do not use the abdominal wall as a fulcrum, so that the abdominal wall is less traumatised, resulting in less bruising and pain.
For any cancer surgery, the primary objective is to safely achieve cure. Other objectives such as recovery time, cost and cosmesis must be secondary. While the published data has been almost universally encouraging, so far there have been no prospective randomised controlled trials validating robotic surgery with reference to these objectives in gynaecological oncology.
Endometrial cancer
The significant benefit of laparoscopic surgery versus open surgery has been convincingly demonstrated by the LACE trial.3 It is likely that the robotic approach will offer similar improvements in outcome for women with endometrial cancer. The more important question is in regard to the comparison of robotic surgery to laparoscopic surgery in endometrial cancer. The recent Cochrane meta-analysis4 concluded that robotic surgery results in less blood loss compared to either laparoscopy or laparotomy. The robotic platform has been shown to provide similar or increased lymph node yields compared to laparoscopic surgery5, and the adequacy of surgery is improved in the case of morbidly obese patients compared to laparoscopic surgery.6 Obesity is a major causative factor for endometrial cancer and the incidence of both is increasing dramatically in Australia. Weinberg’s group7 concluded that robotic surgery becomes increasingly advantageous over laparoscopic surgery for women with endometrial cancer as the potential complexity of the surgery is increased by co-morbidities such as obesity, larger uteri and previous abdominal surgery. The role for robotic surgery in endometrial cancer in Australia is thus likely to increase.
Cervical cancer
The increased surgical finesse, range of motion and three-dimensional vision offered by the robot provides significant advantage to the surgeon especially in the parametrial and ureteric dissection and in preserving the autonomic nerve supply to the bladder when undertaking radical hysterectomy for cervical cancer. Many published reports8-11 have demonstrated the safety and feasibility of robotic radical hysterectomy and pelvic lymph node dissection in the management of early-stage cervical cancer. These reports have consistently demonstrated that robotic surgery is associated with longer operating times than open surgery, but shorter than laparoscopic surgery and with less blood loss than either. Robotic fertility-sparing surgery (radical trachelectomy) for selected cases of early cervical cancer has been described12,13 and is likely to be easier to master than the equivalent vaginal radical trachelectomy. The overall role for robotic surgery for early-stage cervical cancer in Australia is likely to increase initially as more gynaecological oncologists are trained in robotic surgery. However, in the longer term, the incidence of early cervical cancer will diminish due to the effectiveness of screening programs and HPV vaccination.
Ovarian cancer
The management of ovarian cancer requires optimal surgical cytoreduction (debulking) in advanced stage disease and, less commonly, a definitive staging procedure in apparent stage I disease. While robotic surgery can achieve these surgical goals within the pelvis, its limitation is in its relative inability to operate simultaneously and extensively within the abdomen and pelvis. The literature contains only a few case reports and small series. Magrina14 has published a retrospective series of 25 patients undergoing primary surgery performed robotically for ovarian cancer and concludes that limitations of robotic technology will probably prevent its widespread application in patients with ovarian cancer.
Training and accreditation
There is currently no universally recognised training or credentialling requirement for gynaecologists wishing to perform robotic surgery in Australia. As is stands, each hospital currently maintains its own requirements. Nevertheless there has been some consistency. Most hospitals in Australia have, thus far, required a gynaecologist intending to take up robotic surgery to first demonstrate proficiency in advanced laparoscopic procedures, to undertake case observations and/or assisting other surgeons with cases, to practice on a robotic simulator and complete an industry-run standard training module. This training module is a two-day workshop consisting of didactic lectures and hands-on live surgery in an animal laboratory and is run by Intuitive Surgical at various sites in the USA, Europe and Hong Kong. Then the first three robotic surgery cases must be performed with the supervision of an experienced proctor. In order to be recognised by Intuitive Surgical as a robotic surgeon, one must perform a minimum of 20 robotic cases in the first 12 months and 20 cases in every 12 months thereafter.
The learning curve for robotic surgery will be different for each individual surgeon; however, it has been estimated that between 20 and 50 cases are required to obtain proficiency.15 The learning curve continues and individual surgeons report continuous improvements to their technique up to and beyond 100 cases.16 The learning curve will be much steeper for surgeons who are not already proficient in laparoscopic surgery, as they will be learning new surgical techniques at the same time as they are learning to use the robotic system, in contrast to experienced laparoscopists who can apply their existing laparoscopic skills that will be extended and enhanced by the adoption of robotics.
Costs and limitations
There is no doubt that, in the learning phase, robotic surgery cases take longer to set up than the equivalent open or laparoscopic procedure. This can be largely overcome by engaging a consistent team of committed theatre staff, including anaesthetist, surgical assistant, scrub and scout nurses and theatre technicians.
Cost is the major limitation to the introduction and widespread acceptance of robotic surgery in gynaecological oncology practice. The capital outlay for a daVinci system is around $3m, with additional annual service costs. The EndoWrist robotic instruments have a lifetime of ten uses and must thereafter be disposed of, adding around $3000 to the cost of each case. There are currently no HIC item numbers specific to robotic surgery. Many private health insurance funds will cover the cost of the disposable equipment, some will not. The funding arrangements are complex and the business model will be different for each institution. Nevertheless, the cost has not been a disincentive to the introduction of robotic surgery in my own private practice where I have completed over 100 cases. Dr Martin Oehler, at Royal Adelaide Hospital, has introduced a successful robotic gynaecologic oncology program into a public hospital system, working within these financial constraints, and has completed more than 150 cases.17
The future
One of the main barriers to the implementation of robotic surgery in gynaecological oncology practice in Australia has been limitation of access, as few hospitals had purchased robotic systems and those that did were often already fully utilised by urologists. This is changing rapidly and there are currently five robots in Melbourne alone, with 12 in Australia overall, three in New Zealand and more on the order books. The second barrier has been the cost. Every nation’s health budget is under strain and it is important that all new technologies be justified with respect to the cost/benefit equation. A number of US18-20 studies and at least one21 European analysis of robotic surgery costs have concluded that robotic surgery is more expensive than either laparoscopic or open surgery, however, the difference is minimised when a high volume of cases is undertaken robotically in order maximise amortisation of the capital outlay.20 These analyses have not taken into account the societal cost of surgery, such as the cost of time off work to the employer, to the family and to the individual. While the cost modelling is unlikely to be directly transferable to the Australian healthcare system, the conclusions are likely to be similar. Nevertheless, at some time in the future, a competitor may enter the marketplace as an alternative robotic system is developed. Competition will dissolve the current monopoly and inevitably lead to reductions in cost.
It is almost inevitable that successive generations of robots will become more compact, even easier to use and incorporate other technologies, such as ultrasound probes or other imaging modalities to identify anatomy deep to the surgical field. A single port robot system has been produced in prototype form only and may offer even greater benefits.22
Robotic surgery is feasible and safe for the management of endometrial cancer and early cervical cancer. Data from further well-constructed trials will emerge to better define its role in gynaecological cancer surgery. There is likely to be a steady and consistent uptake of robotic surgery by gynaecological oncologists in Australia as more hospitals acquire the technology.
References
- www.intuitivesurgical.com/products/davinci_surgical_systems/#components.
- Manolitsas TP, McCartney AJ. Total laparoscopic hysterectomy in the management of endometrial carcinoma. J Am Assoc Gynecol Laparosc.2002 Feb;9(1):54-62.PubMed PMID: 11821607.
- Janda M, Gebski V, Brand A et al. Quality of life after total laparoscopic hysterectomy versus total abdominal hysterectomy for stage I endometrial cancer (LACE): a randomised trial. Lancet Oncol. 2010 Aug;11(8):772-80. Epub 2010 Jul 16. PubMed PMID: 20638899.
- Lu D, Liu Z, Shi G, et al. Robotic assisted surgery for gynaecological cancer. Cochrane Database Syst Rev. 2012 Jan 18;1:CD008640.PubMed PMID: 22258988.
- Seamon LG, Cohn DE, Henretta MS et al. Minimally invasive comprehensive surgical staging for endometrial cancer: Robotics or laparoscopy? Gynecol Oncol. 2009 Apr;113(1):36-41. Epub 2009 Jan24. PubMed PMID: 19168206.
- Gehrig PA, Cantrell LA, Safer A et al.What is the optimal minimally invasive surgical procedure for endometrial cancer staging in the obese and morbidly obese woman? Gynecol Oncol. 2008; 111(1) 41-5
- Weinberg L, Rao S, Escobar PF. Robotic Surgery in Gynecology: An Updated Systematic Review. Obstet Gynecol Int 2011. 2011 852061 Epub 2011 Nov 28.
- Boggess JF, Gehrig PA, Cantrell LA et al. A case-control study of robot assisted type III radical hysterectomy with pelvic lymph node dissection compared with open radical hysterectomy. Am J Obstet Gynecol. 2008 Oct;199(4):357.e1-7. PubMed PMID: 18928973.
- Cantrell LA, Mendivil A, Gehrig PA et al. Survival outcomes for women undergoing type III robotic radical hysterectomy for cervical cancer: a3-year experience. Gynecol Oncol. 2010 May;117(2):260-5. Epub 2010 Feb 13. PubMed PMID: 20153886.
- Lowe MP, Chamberlain DH, Kamelle SA, et al. A multi-institutional experience with robotic-assisted radical hysterectomy for early stage cervical cancer. Gynecol Oncol. 2009 May;113(2):191-4. Epub 2009 Feb 26. PubMed PMID: 19249082.
- Fanning J, Fenton B, Purohit M. Robotic radical hysterectomy. AmJ Obstet Gynecol. 2008 Jun;198(6):649.e1-4. PubMed PMID:18538146.
- Geisler JP, Orr CJ, Manahan KJ. Robotically assisted total laparoscopic radical trachelectomy for fertility sparing in stage IB1 adenosarcoma of the cervix. J Laparoendosc Adv Surg Tech A. 2008 Oct;18(5):727-9.PubMed PMID: 18803518.
- Diaz JP, Sonoda Y, Leitao MM et al. Oncologic Outcome of FertilitySparing Radical Trachelectomy versus Radical Hysterectomy for stage1b1 cervical carcinoma. Gynecol Oncol 2008 111 255-260.
- Magrina JF, Zanagnolo V, Noble BN et al. Robotic approach for ovarian cancer: perioperative and survival results and comparison with laparoscopy and laparotomy. Gynecol Oncol. 2011 Apr;121(1):100-5.Epub 2010 Dec 30. PubMed PMID: 21194736.
- Lenihan JP Jr, Kovanda C, Seshadri-Kreaden U. What is the learning curve for robotic assisted gynecologic surgery? J Minim Invasive Gynecol. 2008 Sep-Oct;15(5):589-94. PubMed PMID: 18722971.
- Seamon LG, Fowler JM, Richardson DL et al. A Detailed Analysis of the Learning Curve: Robotic Hysterectomy and pelvic-aortic lymphadenectomy for endometrial cancer. Gynecol Oncol 2009 Aug;114(2): 162-7. Epub 2009 May 9. PubMed PMID 19428096.
- Oehler MK. Robotic surgery in gynaecology and gynaecological oncology: program initiation and operative outcomes at the Royal Adelaide Hospital. ANZJOG. 2011 Apr;51(2):119-24. doi:10.1111/j.1479-828X.2011.01293.x. Epub 2011 Mar 16. PubMedPMID: 21466512.
- Venkat P, Chen LM, Young-Lin N et al. An economic analysis of robotic versus laparoscopic surgery for endometrial cancer: Costs, charges and reimbursements to hospitals and professionals. Gynecol Oncol.2011 Nov 25. [Epub ahead of print] PubMed PMID: 22120176.
- Holtz DO, Miroshnichenko G, Finnegan MO et al. Endometrial cancer surgery costs: robot vs laparoscopy. J Minim Invasive Gynecol. 2010 Jul-Aug;17(4):500-3. Epub 2010 May 23. PubMed PMID: 20547112.
- Barnett JC, Judd JP, Wu JM et al. Cost comparison among robotic, laparoscopic, and open hysterectomy for endometrial cancer. Obstet Gynecol. 2010 Sep;116(3):685-93. PubMed PMID: 20733453.
- van Dam P, Hauspy J, Verkinderen L et al. Are costs of robot-assisted surgery warranted for gynecological procedures? Obstet Gynecol Int.2011;2011:973830. Epub 2011 Sep 18. PubMed PMID: 21941556;PubMed Central PMCID: PMC 3175389.
- Escobar PF, Kebria M, Falcone T. Evaluation of a novel single port robotic platform in the cadaver model for the performance of various procedures in gynecologic oncology. Gynecol Oncol. 2011 Mar;120(3):380-4. Epub 2011 Jan 8. PubMed PMID: 21216452.






Leave a Reply