Few gynaecological conditions cause as much unnecessary fear and confusion for patients as uterine fibroids. We examine the latest thinking on the management of this common pathology.
Leiomyomata (fibroids) of the uterus were among the first specific gynaecological pathologies ever described. Surgical removal of fibroids (myomectomy) was first described at the end of the 19th century, although at that time the mortality from the procedure approached 15 per cent.1 We now recognise that fibroids are extremely common, although an exact prevalence is difficult to provide. Ultrasound studies have described an increasing prevalence as the age of women examined increases, with an overall lifetime incidence of about 50 per cent. However, if histological criteria are used, as many as three quarters of women will have fibroids.2
The aetiology of fibroids is only partially understood. They are clonal, meaning that each individual fibroid has arisen from a single myometrial cell.2 Fibroids are common in women of Afro-Caribbean descent, although the reasons for a racial difference are not clear. It seems likely that multiple factors are involved in regulating the growth of fibroids, in particular the sex steroids progesterone and oestradiol. It may be that the muscle cells that compose fibroids have increased expression of oestrogen receptors.3 Multiparous women are more likely to have fibroids, as are those with a longer menarche-menopause interval. Other conditions associated with an increased risk of fibroids include obesity, polycystic ovary syndrome, diabetes and hypertension.1
Diagnosis of fibroids
Many women will undergo examination and investigation after reporting such symptoms as heavy menstrual bleeding, pelvic ‘pressure’ and, sometimes, an incidental finding of a pelvic mass. In some cases, uterine fibroids are not suspected and are incidentally discovered during investigation of unrelated problems. Ultrasound is the most commonly used diagnostic modality and has both high positive predictive and negative predictive values.4 Ultrasound is very useful in the triage of pelvic tumours, allowing sensitive and specific differentiation from uterine malignancy and ovarian masses. Other modalities, such as magnetic resonance imaging (MRI) and computed tomography imaging, sometimes provide additional information that can assist in planning treatment, such as evidence of effect on the ureters.
There are differing classifications of fibroids, depending on their size and location in the uterus. However, the most clinically useful classification is: submucous (fibroids that distort the uterine cavity); intramural (where there is no component in the uterine cavity and the majority of the fibroid is contained within the uterine wall); and subserous (where the majority of the fibroid is without the uterus, commonly on a pedicle).
Management of uterine fibroids
Since fibroids are manifestly common and most women are completely asymptomatic, the majority of women do not need any form of treatment apart from reassurance. However, when there is a clinical symptom complex associated with the fibroids, it may be necessary to offer treatment. Such clear-cut symptoms include heavy menstrual bleeding or sometimes a sensation of ‘pressure’ within the pelvis. However, there are also associations between the presence of fibroids and either infertility, early pregnancy loss or late pregnancy complications, and sometimes all of these.
Let’s begin with the simpler situation of submucous fibroids. These are unequivocally associated with heavy menstrual bleeding owing to the increase in endometrial surface area and the common presence of vessels coursing over the surface of the fibroid. It is also likely that the normal mechanisms that limit the duration of menstrual periods – coordinated occlusion of the spiral arterioles supplying the endometrium by myometrial contractions – is disrupted. Submucous fibroids are also associated with fertility delays, implantation failure and early pregnancy loss.5 Medical therapy where fertility is a consideration is usually contraindicated, except in acute situations, since treatment with progestins or GnRH analogues is incompatible with pregnancy. The most useful therapy is hysteroscopic resection. Larger fibroids may be managed with pre-operative GnRH analogues to help reduce the size and vascularity of the fibroid.1 Hysteroscopic surgery has been complicated by intrauterine adhesions and perforation, so careful counselling and consideration is required.
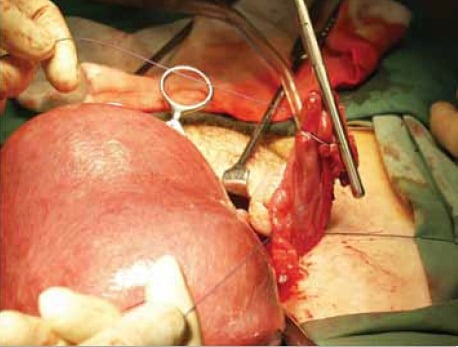
Figure 1. Abdominal hysterectomy for a large, fibroid uterus. Massive uterine enlargement from uterine fibroids is one of the commoner indications for abdominal hysterectomy in the era of minimally invasive surgery.
Decision-making with intramural and subserous fibroids can be more difficult. Therapies can be divided into medical, interventional imaging and surgical. Medical therapies include GnRH agonists and antagonists, selective oestrogen receptor modulators (SERMS), the levonorgestrel-releasing intrauterine system (Mirena®) and more experimental therapies, including mifepristone and cabergoline.
Medical therapy of uterine fibroids
Prolonged use of GnRH agonists such as goserelin (Zoladex™), leuproline (Lucrin™), and naferelin (Synarel™) has been reported to reduce the volume of fibroids. However, the side effects of prolonged GnRH agonist therapy severely limit their use. More prolonged therapy can be maintained with ‘add back’ of the oestrogen β-receptor (ERβ) agonist tibolone.6 Mirena is commonly successful in reducing menstrual flow, but the effect on the volume of fibroids is minimal.1
Interventional imaging
Two interventional techniques have entered common use in the management of fibroids – uterine artery embolisation (UAE) and high-intensity focused ultrasound (HIFU). Embolisation of the uterine arteries aims to reduce the volume and clinical effects of fibroids while preserving the uterus. There are various techniques for the procedure, but in all cases a unilateral femoral artery catheter is passed by an interventional radiologist and selective catheterisation of the uterine arteries or branches identified as supplying fibroids is then undertaken. The vessels are then embolised with (usually) non-absorbable materials such as polyvinyl alcohol (PVA) particles. When used to treat fibroids associated with heavy menstrual bleeding, the results are good in the short term.7
There have been a number of concerns raised about the effect of UAE on subsequent pregnancy, however. Although studies are limited, a number of pregnancies have been reported in women who have undergone UAE and these are characterised by higher rates of preterm birth, malpresentation and caesarean delivery.7 However, it is difficult to know whether these women would have been able to become pregnant without the procedure and, if they had, how these pregnancies would have progressed.
HIFU, where high-intensity ultrasound has been used to treat fibroids, can usually only target one fibroid at a time and has been associated with burns to the skin. More data are required before the value of HIFU can be fully assessed.
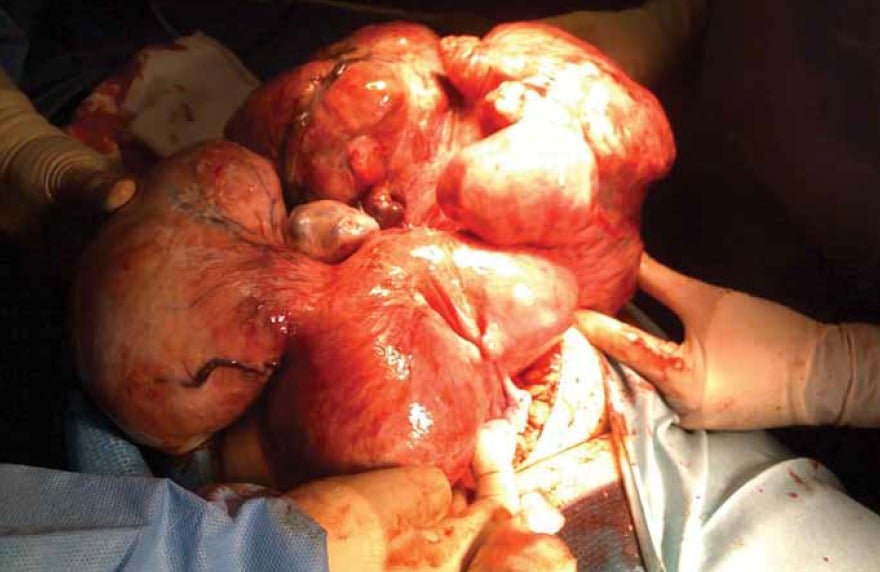
Figure 2. A massive fibroid uterus at hysterectomy. Preservation of the ovaries in this situation can be difficult for even the most experienced surgeon.
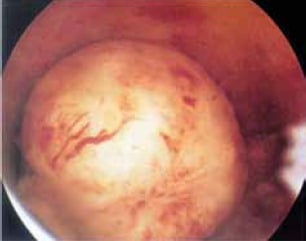
Figure 3. A submucous fibroid. Note the large vessels coursing across the surface. Hysteroscopy surgery is the only effective method of dealing with this type of fibroid while still preserving fertility.
Surgical treatment of fibroids
Two surgical therapies are available – hysterectomy and myomectomy. Hysterectomy is obviously a definitive treatment, but non-surrogate pregnancy afterwards is not possible. It is certainly not an option for younger women desiring preservation of fertility. In general, vaginal hysterectomy is the preferred route and large uterine volume is not a contraindication.8 However, laparoscopic hysterectomy (of whatever level) may be associated with reduced blood loss,and is certainly superior if removal of the adnexal structures is required at the same time.
Myomectomy aims to preserve the uterus and, with it, fertility in general terms. It can be performed by the abdominal or laparoscopic route. Laparoscopic myomectomy is associated with shorter inpatient course, more rapid recovery and return to normal function and reduced need for transfusion.8 However, there appear to be no benefits with respect to subsequent chance of pregnancy and complications thereof.
Uterine fibroids and infertility
The Australasian CREI Consensus Expert Panel on Trial evidence (ACCEPT) in 2011 published a consensus statement on fibroids in fertility, which now forms a College statement.9,10 When infertility is an issue the group recommend using MRI, sonohysterography or hysteroscopy for determining whether there is uterine cavity involvement by the fibroid. The group reported that subserosal fibroids did not affect fertility outcomes, while intramural fibroids may be associated with reduced fertility and an increased miscarriage rate. There was however insufficient evidence to show that myomectomy for intramural fibroids improved fertility outcomes. Submucosal fibroids are associated with reduced fertility and increased miscarriage and hysteroscopic myomectomy is likely to improve outcomes.
The group recommended myomectomy for infertile women if: a woman is infertile and has submucosal fibroids, or a woman has symptomatic fibroids, or a couple has multiple failed cycles of assisted reproductive technology and the female partner has intramural fibroids. The group also concluded that medical treatment delayed efforts to conceive and was not recommended, other than using GnRH analogues in the short term to correct preoperative anaemia or reduce fibroid volume and that treatments such as uterine artery embolisation, MRI-guided focused ultrasound surgery and radiofrequency ablation should only be used in the setting of approved clinical trials.
Fibroids and cancer
Patients often ask if uterine fibroids should be removed because of the risk of cancer. Leiomyosarcoma is rare, particularly in premenopausal women, with only 0.1 per cent of uterine smooth muscle tumours being malignant leiomyosarcomas.11
A rapidly enlarging myoma in a postmenopausal woman has a higher likelihood of malignancy, with leiomyosarcoma being reported in 1.4 per cent to 1.7 per cent of women undergoing hysterectomy in the sixth or seventh decade of life.12 In the vast majority of cases leiomyosarcoma arises de novo, not from pre-existing fibroids, although rare case reports do exist of leiomyosarcoma arising within fibroids.11,12
Summary
Uterine fibroids are incredibly common and should almost be considered a normal part of the anatomy for women aged over 40 years. In the majority of cases, fibroids are completely incidental findings and women are asymptomatic. When symptoms are present, a decision needs to be made about whether fertility preservation, either in the short or long term, is required as this is a major driver of decision-making. Conservative medical therapies are usually incompatible with pregnancy. If the symptoms are related to recurrent pregnancy loss or fertility delays, surgical treatment is usually indicated. Other treatments, such as UAE, may have a role, but data are limited when compared to myomectomy in these circumstances. Definitive treatment is by hysterectomy, with its obvious potential disadvantages. As always, the treatment has to be individualised to the patient.
References
- Salman T, Davis C. Uterine fibroids, management and effect on fertility. Curr Opin Obstet Gynecol 2010; 22: 295-303.
- Okolo S. Incidence, aetiology and epidemiology of uterine fibroids. Best Pract Res Clin Obstet Gynaecol 2008; 22: 571-588.
- Kovaacs K, Oszter A, Goecze P, et al. Comparative analysis of cyclin D1 and oestrogen reception (a and b) levels in human leiomyomata and adjacent myometrium. Mol Hum Reprod 2001; 7: 1085-1091.
- Somigliana E, Vercellini P, Daguati R, et al. Fibroids and female reproduction: a critical analysis of evidence. Hum Reprod Update 2007; 13: 465-476.
- Pritts E. Fibroids and infertility: a systematic review of the evidence. Obstet Gynecol Surv 2001; 56: 483-491.
- Palomba S, Orio F, Russo T, et al. Long-term effectiveness and safety of GnRH agonist plus raloxifene administration in women with uterine leiomyomas. Hum Reprod 2004; 19: 1308-1314.
- Berkane N, Moutafoff-Borie C. Impact of previous uterine artery embolization on fertility. Curr Opin Obstet Gynecol 2010: 22: 242-247.
- Candiani M, Izzo S. Laparoscopic versus vaginal hysterectomy for benign pathology. Curr Opin Obstet Gynecol 2010; 22: 304-308.
- roon B, Johnson N, Chapman M, et al. Fibroids in infertility-consensus statement from ACCEPT (Australasian CREI Consensus Expert Panel on Trial evidence). ANZJOG 2011; 51: 289-295.
- RANZCOG College Statement Fibroids in infertility (C- GYN 27) 2011.
- Yanai H, Wani Y, Notohara K, et al. Uterine leiomyosarcoma arising in leiomyoma: Clinicopathological study of four cases and literature review. Path Int 2010; 60: 506-509.
- Munro MG, Uterine leiomyomas, current concepts: Pathogenesis,impact on reproductive health, and medical, procedural and surgical management. Obstet Gynecol Clin N Am 2011; 38: 703-731.







Leave a Reply