This article explores the risk factors for stillbirth in diabetic pregnancy, the underlying pathophysiology of diabetes in pregnancy relating to potential mechanisms of fetal death and the important roles of optimised glycaemic control as well as careful fetal surveillance in reducing stillbirth risk.
Diabetes is now a common problem in pregnancy, with Australian data revealing that 1.7 per cent of pregnancies are complicated by pre-existing diabetes and 6.9 per cent have a diagnosis of gestational diabetes (GDM).1 As both pre-existing diabetes and GDM are associated with increased adverse outcomes for both mother2 and baby3, and now almost one pregnancy in ten is affected, diabetes imposes a very heavy burden on providers of maternity care. The increased rate of stillbirth and neonatal death in the offspring of mothers with pre-existing diabetes is well recognised, with rates of stillbirth increased by up to five times compared to non-diabetic pregnancies.4-11 Although an area of controversy, GDM may also be associated by an estimated 25 per cent increase in stillbirth.12
Stillbirth risk factors in pre-existing diabetic pregnancy
In the UK, the offspring of women with type 1 and type 2 diabetes were five times more likely to be stillborn, with 80 per cent of the stillborn babies being structurally normal.6,11 Together with congenital malformations, the occurrence of pregnancy complications such as intrauterine growth restriction, pre-eclampsia, umbilical cord problems, acute asphyxia, placental abruption and intrauterine infections explain about 50 per cent of the stillbirths, with 50 per cent being unexplained.7 Diabetic nephropathy, smoking and low socioeconomic status have been reported to be risk factors.7 In a French study of perinatal mortality of 289 women with type 1 diabetes, those who received preconception care had a perinatal mortality rate of 0.7 per cent compared to 8.1 per cent for those that did not, suggesting glycaemic control at conception and during the first trimester are critically important.10 Other European studies have revealed that the better the glycaemic control in pregnancy, the lower the risk of perinatal death.5,13,14 For example, the mean HbA1c was higher in women with pregnancies complicated by stillbirth, compared to uncomplicated diabetic pregnancies.5 For this reason, it has been concluded that, ‘the single most important factor to reduce the risk of stillbirth is to achieve and maintain good glycaemic control during pregnancy.’5
Gestational diabetes and stillbirth
GDM and type 2 diabetes share the same underlying mechanism – failure of the islet beta-cells to compensate for insulin resistance – and can be considered different phases of the same condition.15 As type 2 diabetes is clearly associated with increased stillbirth rates, one might expect that GDM would be as well, although, evidence to show this is not strong. The well-known Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study did not show any increase in perinatal mortality with hyperglycaemia of lesser magnitude than overt diabetes.16 Also, large population-based studies have generally provided reassurance, reporting that the risk of stillbirth in pregnancies affected by GDM is not increased. However, it has recently been suggested that such analyses may be flawed. This is because, although most pregnancy outcome cohorts begin at 20 weeks gestation, the pregnancy must continue beyond 28 weeks for the screening procedures for GDM (as compared to types 1 and 2 diabetes, which are almost always diagnosed by 20 weeks). When national data from the USA were re-analysed and the analysis was restricted to pregnancies that progressed beyond 28 weeks gestation, the authors found that GDM was indeed associated with an increase in the odds for stillbirth (adjusted OR 1.25, 95 per cent CI 1.11, 1.41).12 In light of this new evidence that GDM may be associated with a significant increase in the odds for stillbirth and in view of the increasing incidence of GDM, especially since the diagnostic criteria for GDM are in a state of flux, it is important to review this important question. In doing this, other non-glycaemic factors that may also impact on adverse outcomes in GDM pregnancy such as obesity, hyperlipidaemia and inflammation also need to be considered.15
It should also be noted that HAPO demonstrated an almost linear relationship with increasing levels of hyperglycaemia and a range of other adverse pregnancy outcomes.16 The results of other large studies have shown the diagnosis and management of gestational diabetes is associated with improved outcomes.15,17
Pathophysiology of maternal diabetes on the fetus
The pathophysiology of diabetic effects on the fetus in later pregnancy are summarised by the ‘Pederson hypothesis’: maternal hyperglycaemia results in fetal hyperglycaemia, which in turn overstimulates the fetal pancreatic beta cells to cause fetal hyperinsulinaemia.18 Glucose is the main source of energy for the fetus. It crosses the placenta by non-insulin mediated, but concentration gradient dependent, diffusion processes facilitated by hexose transporters. In addition to transferring intact glucose molecules, placental glycolysis yields lactate, which is another source of fetal energy substrate. The placenta has limited capacity to buffer glucose transfer by metabolism into glycogen.
Fetal hyperinsulinaemia, together with the enhanced fetal nutrient supply, drives high rates of fetal growth, deposition of subcutaneous fat and storage of glycogen in the liver. These quite marked effects are associated with increased metabolic rates that may provoke fetal hypoxia. A chronic hypoxia could be further aggravated later in pregnancy with placental changes induced by diabetes. Hypoxic fetuses have been found to have elevated levels of erythropoietin (EPO) in blood and amniotic fluid, just as happens in adults.19,20 Supportive of the concept that hypoxia occurs in fetuses of diabetic mothers is a strong correlation between maternal HbA1c levels in late pregnancy and umbilical cord EPO concentrations.21
In pre-existing type 1 diabetic pregnancies, neonatal echocardiography reveals signs of cardiomyopathy (with septal hypertrophy and heart enlargement) in up to 40 per cent of newborns.7 Although the cause of this fetal/neonatal diabetic cardiomyopathy is unknown, increased nutrient supply and hyperinsulinism are likely to be involved. An almost certain consequence will be increased myocardial oxygen consumption that, in the setting of fetal hypoxia, may increase the susceptibility of the heart to arrhythmias.
Further evidence implicating the heart in stillbirth of diabetic pregnancy is provided by studies of the peptide B-type natriuretic protein (BNP), a known product of cardiac muscle cells in response to stress. In the fetus, BNP is known to be a vasodilator in placental vessels, among other functions.22 Studies of cord blood BNP levels, as well as Troponin T (a marker of myocardial damage) correlate with poor maternal glycaemic control in pregnancy.7
Management of diabetes in pregnancy
Since maternal diabetes is so strongly associated with stillbirth, strategies of pre-pregnancy care, multidisciplinary pregnancy care, timing of delivery and intrapartum care are vital in optimising neonatal outcomes and reducing perinatal mortality.23
Diabetes is perhaps the foremost example of the benefits of pre-pregnancy care on perinatal outcomes.24 Elevated HbA1c levels in early pregnancy are strongly associated with adverse outcomes of pregnancy, including pregnancy loss and fetal malformations.25 The principles of pre-pregnancy care have been well described by Temple25 (see Box 1). While the evidence is convincing that pre-pregnancy care reduces the risk of congenital malformations and there are very good indications it also reduces perinatal mortality,10 the same benefits have not been demonstrated for other adverse outcomes, such as premature delivery and pre-eclampsia.25
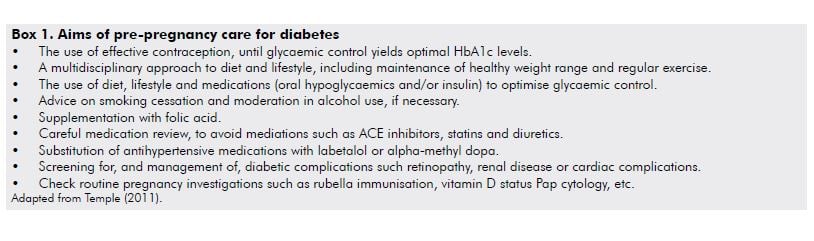
For the majority of women with pre-existing diabetes – either type 1 or, increasingly, type 2 – good-quality pregnancy care in a multidisciplinary team will minimise the risk of perinatal complications (see Box 2).3 Optimised glycaemic control will help to reduce stillbirth and other complications, however, even the tightest glycaemic control the risk of stillbirth and neonatal death remains increased.7 Additional important principles of diabetic care in pregnancy include: regular fetal surveillance by ultrasound, both to detect congenital abnormalities in the mid-trimester and to screen for growth restriction or macrosomia in the third trimester; and, attention to patterns of fetal movement including kick charts, with recourse to cardiotocography (CTG) assessment if indicated. Taking such an approach the stillbirth rate has been reduced from two per cent (1993–99) to 0.7 per cent (2000–09) in a centre of expertise in diabetic pregnancy in Denmark.7

Conclusions
Diabetes is a common and important cause of stillbirth and perinatal death, among other adverse outcomes of pregnancy. Both pre-pregnancy care and close attention to the optimisation of glycaemic control during pregnancy are critical to reducing the risk of stillbirth. This will require a multidisciplinary approach and include careful fetal surveillance and timely delivery. There are new data to suggest that GDM, previously supposed to have little effect on stillbirth, may actually increase the odds for this important and distressing complication. Similar principles apply to the management of GDM as apply to pre-existing types 1 and 2 diabetes, but the screening criteria for GDM are evolving and further study will be required over coming years.
References
- Li Z, Zeki R, Hilder L, Sullivan E. Australia’s mothers and babies 2010. Perinatal statistics series no. 27. Cat. no. PER 57. Canberra: AIHW National Perinatal Epidemiology and Statistics Unit, 2012.
- Hawthorne F. Maternal complications in diabetic pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25: 77-90.
- Hawdon J. Babies born after diabetes in pregnancy: what are the short- and long-term risks and how can we minimise them? Best Pract Res Clin Obstet Gynaecol 2011; 25: 91-104.
- Evers I, de Valk H, Visser G. Risk of complications of pregnancy in women with type 1 diabetes: nationwide prospective study in the Netherlands. BMJ 2004; 328: 915.
- Jensen D, Damm P, Moelsted-Pederson, et al. Outcomes in type 1 diabetic pregnancies: a nationwide, population-based study. Diabetes Care 2004; 27: 2819-23.
- Macintosh M, Fleming K, Bailey J, et al. Perinatal mortality and congenital anomalies in babies of women with type 1 or type 2 diabetes in England, Wales, and Northern Ireland: population-based study. BMJ 2006; 333: 177.
- Mathieson E, Ringholm L, Damm P. Stillbirth in diabetic pregnancies. Best Pract Res Clin Obstet Gynaecol 2011; 25: 105-111.
- Shand A, Bell J, McElduff A, et al. Outcomes of pregnancies in women with pre-gestational diabetes mellitus and gestational diabetes mellitus: a population-based study in New South Wales, Australia, 1998-2002. Diabetic Med 2008; 25: 708-15.
- Colstrup M, Mathieson E, Damm P, et al. Pregnancy in women with type 1 diabetes: Have the goals of St. Vincent declaration been met concerning foetal and neonatal complications? J Matern Fetal Neonatal Med; 26: 1682-6.
- 10 Diabetes and Pregancy Group, France. French multicentric survey of outcome of pregnancy in women with pregestational diabetes. Diabetes Care; 26: 2990-3.
- Confidential Enquiry in Maternal and Child Health. Confidential enquiry into maternal and child health: pregnancy in women with type 1 and type 2 diabetes in 2002-3. England, Wales, and Northern Ireland: CEMACH, 2005.
- Hutcheon J, Kuret V, Joseph K, Sabr Y, Lim K. Immortal time bias in the study of stillbirth risk factors: the example of gestational diabetes. Epidemiol 2013; 24: 787-90.
- Ekbom P, Damm P, Feldt-Rasmussen B, et al. Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care 2001; 24: 1739-44.
- Nielsen L, Pederson-Bjergaard U, Thorsteinsson B, et al. Hypoglycaemia in pregnant women with type 1 diabetes: predictors and role of metabolic control. Diabetes Care 2008; 31: 9-14.
- Nolan C. Controversies in gestational diabetes. Best Pract Res Clin Obstet Gynaecol 2011; 25: 37-49.
- Metzger B, Lowe L, Dyer A, et al. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med 2008; 358: 1991-2002.
- McIntyre HD, Oats J. Gestational diabetes needs to be managed. MJA 2013; 198: 78-9.
- Pedersen J. The pregnant diabetic and her newborn. Problems and management. Copenhagen: Munksgaard, 1967. Quoted in: Arulkumaran S (ed.): Diabetes in Pregnancy. Best Pract Res Clin Obstet Gynaecol 2011; 25.
- Buescher U, Hertwig K, Wolf C, et al. Etythropoietin in amniotic fluid as a marker of chronic fetal hypoxia. Int J Gynaecol Obstet 1998; 60: 257-263.
- Teramo K, Kari M, Eronen M, et al. High amniotic fluid erythropoietin levels are associate with an increased frequency of fetal and neonatal morbidity in type 1 diabetic pregnancies. Diabetologia 2004; 47: 1695-1703.
- Widness J, Teramo K, Clemons G, et al. Direct relationship of antepartum glucose control and fetal erythropoietin in human type 1 (insulin-dependent) diabetic pregnancy. Diabetologia 1990; 33: 378-83.
- Cameron V, Ellmers L. Natriuretic peptides during development of the fetal heart and circulation. Endocrinology 2003; 144: 2191-4.
- McPherson E. Discovering the cause of stillbirth. Curr Opin Obstet Gynecol 2013; 25: 152-6.
- Nelson-Piercy C. Pre-pregnancy counselling. Curr Opin Obstet Gynecol 2003; 13: 273-80.
- Temple R. Preconception care for women with diabetes: is it effective and who should provide it? Best Pract Res Clin Obstet Gynaecol 2011; 25: 3-14.







Leave a Reply