Urodynamic assessment before surgery for pelvic organ prolapse – why Australasian gynaecologists choose to use it.
The management of more advanced and symptomatic pelvic organ prolapse (POP) is far more complicated than merely identifying the cause of a patient complaint of a bulge and arranging surgery. It involves the interpretation of possible pathogenesis and all relevant symptoms, signs, POP and intercurrent diagnoses. There may still be a role for conservative management (for example, pessary) for a time. Surgical options need clear aims and the best available evidence-based techniques, with the principle primum non nocere (first do no harm) foremost in the clinician’s mind as benign pathology is involved.
The distortional and pressure effects of uterovaginal prolapse (see Figure 1) can have significant impact on other pelvic organs, particularly the lower urinary tract and anorectum. As part of this more informed approach to POP surgery, the need for a more comprehensive assessment of the interaction between POP and lower urinary tract function has been increasingly recognised. Up to 70 per cent of surveyed gynaecologists in Australia and New Zealand would seek urodynamic assessment prior to major prolapse surgery.1 Goals and outcomes for POP interventions, in terms of lower urinary tract function, can be determined. Surgeons can tailor the interventions appropriately, duly counsel and obtain consent from their patients, with the minimisation of adverse functional outcomes. Effects of POP interventions on anorectal dysfunction are, unfortunately, less predictable.
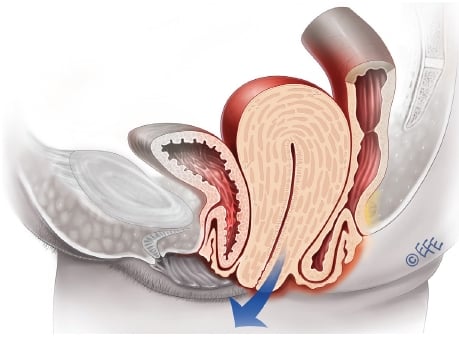
Figure 1. Uterine prolapse, cystocele and rectocoele. (Illustration available at www.medicalartbank.com © Dr Levent Efe, CMI.)
Tertiary clinical assessment
A common scenario is that the urodynamics, formally defined as the ‘functional study of the lower urinary tract2, follows previous primary practitioner and specialist clinical assessments. It is desirable that a mid-stream urine (MSU) is performed prior to urodynamics to eliminate urinary tract infection (UTI), with recurrent UTI (three or more proven UTIs in the previous 12 months) a common comorbidity with symptomatic POP. The tertiary clinical assessment as part of the urodynamics should be as comprehensive as possible. In addition to specific vaginal prolapse symptoms2, potential prolapse-related urinary tract, anorectal and sexual dysfunction symptoms might be revealed. The more* or most common** of such symptoms, in the author’s experience, are listed in Table 1.
| Potential prolapse-related symptoms | |
|---|---|
| Vaginal prolapse | Bulge**, pelvic pressure**, low (sacral) backache* |
| Urinary tract | Frequency**, recurrent UTI**, incomplete emptying**, slow stream* |
| Ano-rectal | Incomplete defecation**, digitation*, rectal urgency* |
| Sexual | Dyspareunia*, vaginal laxity* |
| Other possible associated symptoms | |
| Urinary incontinence** | Stress , urge, postural, nocturnal, coital |
| Bladder storage* | Urgency, nocturia |
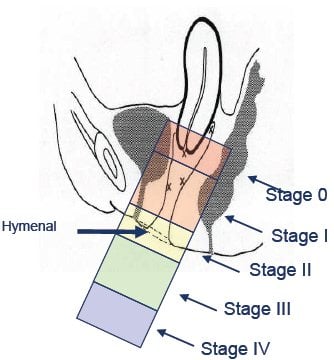
igure 2. Stage for POP – I-IV.2,3
The tertiary clinician should opine in regards to the stages and components of POP present using the internationally recognised quantification (POP-Q)2,3 schema (see Figure 2). Unlike the examination for the sign of stress incontinence (presenting bladder volume comfortably full), which will generally precede the examination for POP, all examinations for POP should be performed with the woman’s bladder empty (and if possible an empty rectum).2 The urodynamic report should include relevant findings from other examinations: external (vulval, perineal, urethral, perianal); vaginal (Sims/Graves speculum); bimanual (uterine, adnexal, other pelvic pathology); and abdominal/neurological (as indicated).
Urodynamic testing
The clinical sequence of (urodynamic) testing2 involves a woman attending with a comfortably full bladder, assessment for stress incontinence (fuller bladder), free (no catheter) uroflowmetry and post-void residual urine volume (PVR) measurement, assessment for prolapse (bladder empty) prior to filling and voiding cystometry. Foci of this tertiary assessment are the relationship of any POP with voiding function; evaluating urinary incontinence and other lower urinary tract symptoms; eliminating other intercurrent pathology with the assistance of imaging; presenting relevant diagnoses; and suggesting possible management strategies.
POP and voiding function
POP will often have a negative impact on voiding function. The effect of uterine prolapse, cystocele or vaginal vault descent is more a ‘kinking’ one with that a rectocoele more a ‘pressure’ effect on bladder outflow through the urethra. The two main basic parameters of voiding function are uroflowmetry and the PVR. In the above clinical sequence of testing2, the uroflowmetry precedes the PVR.
Uroflowmetry
Uroflowmetry is the study of urine flow over time to achieve urine flow rates (see Figure 3a), maximum (MUFR – Qmax) and average (AUFR – Qave) for variable voided volumes. These data can be referenced to nomograms5, which plot the dependency of urine flow rates on voided volume (see Figure 3b). Under the tenth centile of the nomogram5 for the MUFR and average AUFR, on repeated measurement, would be deemed to be abnormally slow. Most commercial uroflowmeters are accurate once calibrated.
Postvoid residual
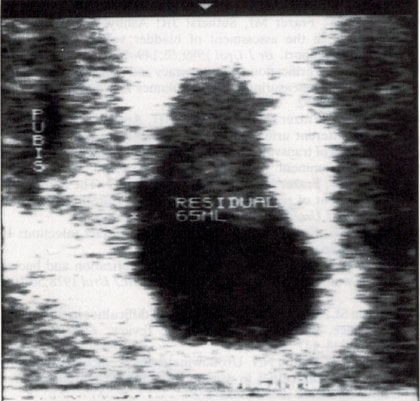
PVR can always be measured, following primary or specialist care assessment, as part of a renal tract ultrasound looking for reasons behind recurrent UTI or other urinary tract symptoms. Most convenient tertiary assessment is by transvaginal6 or translabial ultrasound.7 As most PVR readings still tend to be low, the technical limitations of transabdominal ultrasound makes this less accurate. Urethral catheterisation (most effective by short plastic catheter) is a less accurate and more invasive alternative. Another advantage of ultrasound is that the PVR can be checked by an ‘immediate’ second attempt at voiding (see Figures 4a and 4b).8
It has been shown that increasing stage POP will lead to increasing PVR8 and slower urine flow.9 More sophisticated testing, voiding cystometry, is performed to determine the function of the bladder (detrusor) musculature during voiding. A chronic PVR of over 30ml10 has been shown to be associated with a significant increase in the prevalence of recurrent UTI, a most common clinical association of symptomatic POP. Surgical cure of POP can generally be expected to be associated with improved voiding parameters (little or no PVR; improved urine flow) and symptomatic function and a reduction in the prevalence of recurrent UTI.
Incontinence and other lower urinary tract symptoms
Apart from POP, voiding dysfunction and recurrent UTI, the other three most common diagnoses (prevalence of at least ten per cent in women presenting for tertiary assessment including urodynamics with symptoms of lower urinary tract dysfunction2) in pelvic floor dysfunction are urodynamic stress incontinence (USI), detrusor (bladder) overactivity and bladder oversensitivity. Each of these can occur concurrently with POP, again influencing expectations and management including patient counselling.
USI and POP
USI is defined2 as ‘involuntary leakage, associated with increased intra-abdominal pressure, in the absence of a detrusor (bladder) contraction’. Establishing this conclusively requires the urodynamic investigation of filling cystometry though the sign of clinical stress leakage2 or leakage that occurs simultaneously with coughing or other causes of raised intra-abdominal pressure is strongly suggestive. Sometimes POP can conceal this sign. The occult stress incontinence2 might only be revealed with POP reduction, better preoperatively, when appropriate counselling can occur, rather than postoperatively when it can create patient dissatisfaction as a POP problem is replaced by an incontinence problem.
A clear diagnosis of USI may require counselling on the need for a continence procedure at the time of a proposed POP procedure. This is a very common occurrence.
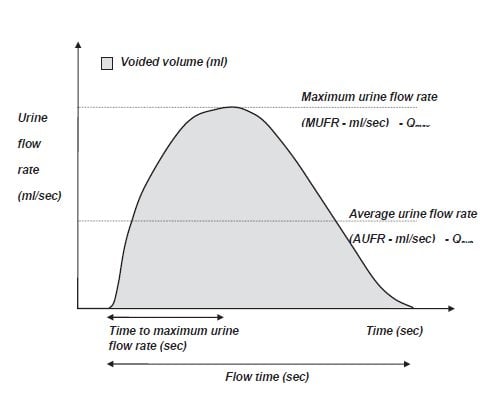
Figure 3a. A schematic representation of urine flow over time.2
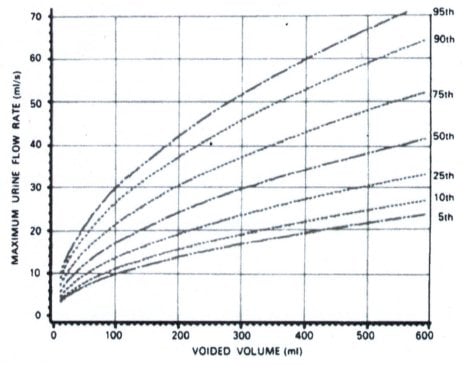
Figure 3b. The Liverpool nomogram for the maximum urine flow rate in women2,5 (under the tenth centile on repeat measurement can be regarded as abnormally slow).
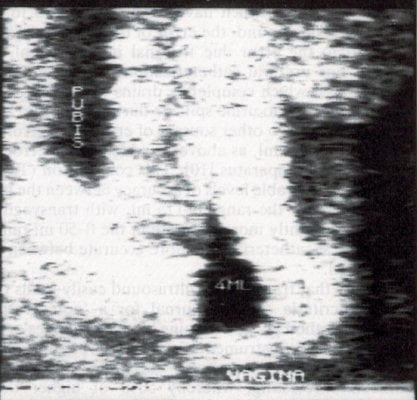
Figures 4a, 4b. An image of postvoid residual of 65ml by transvaginal ultrasound6, reducing to 4ml with a subsequent attempt at voiding.
Detrusor overactivity, bladder oversensitivity and POP
Detrusor (bladder) overactivity is defined2 as ‘the presence of involuntary bladder (detrusor) contractions during filling cystometry’.Bladder oversensitivity is defined1 as ‘increased perceived bladder sensation during bladder filling and decreased bladder (maximum cystometric) capacity in the absence of any abnormal increases in bladder pressure’. Both of these diagnoses, generally associated with such symptoms as nocturia, urgency, urge incontinence and frequency, rely on filling cystometry to confirm. Figure 4 shows a schematic diagram of filling cystometry showing detrusor overactivity. The different lines of the trace show filling volume; Pvescm H20 or the bladder pressure during filling; Pdetcm H20 or the detrusor (true bladder) pressure (Pves – Pdet) during filling; Pabd cm H20 or the abdominal (generally rectal) pressure during filling; and flow rate (ml/sec).
These diagnoses may occur in association with POP and it’s important to know they exist, though there is no clear relation with POP. From a preoperative counselling point of view prior to prolapse surgery, one could suggest to a patient that anatomical restoration might see some relief though perhaps not cure, with ongoing medical treatment possibly necessary.
Imaging and intercurrent pathology
Imaging, particularly by ultrasound, has become an extremely important addition to urodynamic assessment. Many women will have already had the benefit of a pelvic ultrasound prior to the urodynamics. Mention, however, has already been made of the very important use of transvaginal or translabial ultrasound to assess for postvoid residuals. Other uses of the same imaging are to assess: uterine version; bladder neck/urethral morphology/mobility pre- and postoperatively; and descent of pelvic organs.
For women who have not had ultrasound imaging prior to urodynamic testing, there can be an eight per cent detection of significant pathology, not otherwise likely to be detected clinically and with the potential to alter management. These include2:
- intercurrent pelvic pathology (uterine especially large or fibroid uteri, endometrial pathology, adnexal pathology);uteri, endometrial pathology, adnexal pathology);
- bladder abnormalities (tumour, foreign body); and
- urethral abnormality (for example, diverticulum).
More sophisticated analysis of soft tissue pelvic floor/levator defects would generally require the use of 3D or 4D ultrasound.2,11
Radiological studies – such as videocystourethrography (VCU)2 or the synchronous radiological screening of the bladder and abdominal pressures during filling cystometry2 – are less routinely used than they were 20 years ago. Intravenous urography (IVU)2, often by computed tomography, may be used if upper urinary tract pathology is suspected and micturating cystogram (MCU)2 can be useful to evaluate vesico-ureteric reflux, some fistulae and diverticulae.
POP and defecatory function
POP, particularly posterior vaginal compartment prolapse, can impact on defaecatory function. Restoration of the posterior vaginal anatomy in a way where all defects from vaginal vault to perineum are addressed in a systematic way is probably more likely to result in a more satisfactory anatomical and functional result than less comprehensive ad hoc and subjective approaches. Particular care is needed in anatomical restoration of the posterior vaginal compartment to avoid any negative impact on both defaecatory and sexual functions.
In individual cases, there can be a need for additional specialised anorectal functional assessment, which is generally not the province of a gynaecologist or urogynaecologist but rather than of a colorectal surgeon. The most common investigations performed as part of that assessment are:
- Anal ultrasound (endosonography)2, the gold standard investigation in the assessment of anal sphincter integrity. There is a high incidence of defaecatory symptoms in women with anal sphincter defects.
- Defaecography2, to demonstrate normal anatomy of the anorectum as well as disorders of rectal evaluation. Barium paste is inserted rectally over a translucent commode. Rectoceles, enteroceles, rectal intersussception and mucosal prolapse may be diagnosed as well as a spastic pelvic floor (anismus).
- Anorectal physiology: the functional study of this area.
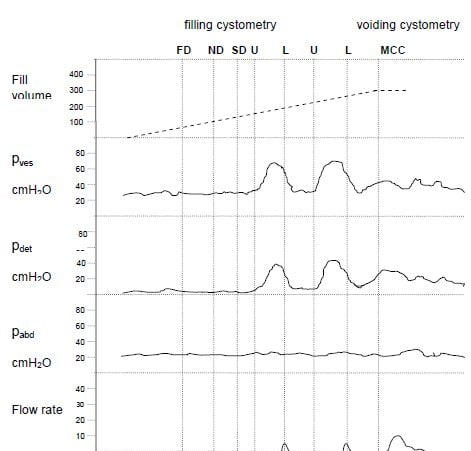
54-year-old female with urgency and frequency. Phasic detrusor activity during filling. Leakage is associated with urgency and detrusor contractions. FD = First Desire to Void, ND = Normal desire to void, SD = Strong desire to void, U = Urgency, L = leakage, MCC = Maximum Cystometric Capacity. U MCC BH / JL 2007 Figure 5. Filling cystometry – detrusor overactivity.2
Diagnoses and possible management strategies
There is a need to base diagnoses for female POP on the correlation between a woman’s symptoms, signs and any relevant diagnostic investigations.2 Other possible (common2 – over ten per cent prevalence) diagnoses include, with the approximate prevalence in women presenting for comprehensive assessment including urodynamics with POP and/or symptoms of lower urinary tract dysfunction:
- urodynamic stress incontinence2 (up to 72 per cent2);
- detrusor overactivity2 (13–40 per cent2);
- bladder oversensitivity2 (10–13 per cent);
- voiding dysfunction2 (14–39 per cent2); and
- recurrent urinary tract infections2 (11 per cent or 19 per cent), depending on whether the cut off is three or two symptomatic and medically diagnosed UTIs in the previous 12 months.
It is therefore not an infrequent scenario with more major prolapse cases (for example, a woman with symptoms of POP, stress and urge urinary incontinence, voiding dysfunction, recurrent UTI and signs of recurrent stage 3 vaginal prolapse) that as many as five diagnoses might be present after comprehensive clinical and urodynamic assessment:
- recurrent UTI (on history);
- stage 3 (on examination) POP (cystocele, rectocoele, vaginal vault descent);
- voiding dysfunction (abnormally slow free uroflowmetry and high PVR);
- USI – partially protected by the obstructive effect of the prolapse and likely to be revealed to an even greater degree by prolapse surgery; and
- mild detrusor overactivity.
The full knowledge and experience of the tertiary assessor is required to propose a triage of management. In the above scenario, one option, after appropriate pre-operative counselling, might be:
- surgical management of POP and voiding dysfunction by her undergoing a sacrospinous colpopexy, anterior and posterior repair, with a concomitant retropubic stress incontinence surgery for the USI;
- ongoing UTI prophylaxis (for example, Hiprex/Vit C both BD) at least till the postoperative visit which would include PVR reassessment; and
- ongoing expectant or medical management of any residual urgency/urge incontinence (detrusor overactivity component) according to symptoms.
Conclusions
The interpretation of possible pathogenesis and all relevant symptoms, signs, POP and intercurrent diagnoses in cases of more advanced and symptomatic POP can require a tertiary assessment including urodynamics by an appropriately skilled assessor. With the increasing awareness of the possible complexity of such cases, it is not surprising that more Australasian gynaecologists are ‘choosing to use it’. They can then tailor the interventions appropriately, duly counsel and consent their patients, with the minimisation of adverse functional outcomes.
References
- Vanspauwen R, Seman E, Dwyer P. Survey of current management of prolapse in Australia and New Zealand. ANZJOG. 2010, 50: 262- 267.
- Haylen BT, Freeman RM, de Ridder D, Swift SE, Berghmans B, Lee J, Monga A, Petri E, Rizk D, Sand P, Schaer G. An International Urogynecological Association (IUGA) – International Continence Society (ICS) Joint Report into the Terminology for Female Pelvic Floor Dysfunction. Neurourology & Urodynamics. 2010, 29:4-20 and International Urogynecology J. 2010, 21:5-26 (dual publication).
- Bump RC, Mattiasson A, Bo K, Brubaker LP, et al. The standardization of female pelvic organ prolapse and pelvic floor dysfunction. Am J Obstet Gynecol. 1996, 175(1):10 -11.
- Yang, A., Mostwin, J., Genadry, R., Sanders, R. Patterns of prolapse demonstrated with dynamic fastscan MRI; reassessment of conventional concepts of pelvic floor weaknesses. Neurourol Urodyn. 1993, 12(4): 310-311.
- Haylen BT, Ashby D, Sutherst JR, Frazer MI and West C Maximum and average urine flow rates in normal male and female populations – the Liverpool nomograms. British Journal of Urology. 1989, 64:21-30.
- Haylen BT, Frazer MI, Sutherst JR and West CR. Transvaginal ultrasound in the assessment of bladder volumes in women. British Journal of Urology. 1989,63:152-154.
- Dietz HP, Velez D, Shek KL, Martin A. Determination of postvoid residuals by translabial ultrasound. Int Urogynecolog J. 2012, 23:1749-1752.
- Haylen BT, Lee J, Logan V, Husselbee S, Zhou J, Law M. Immediate postvoid residuals in women with symptoms of lower urinary tract dysfunction. Obstetrics & Gynecology. 2008, 111:1305-1312.
- Haylen BT, Yang V, Logan V Uroflowmetry: Its current clinical utility for women. Int Urogynecol J. 2008,19:899-903.
- Haylen BT, Lee J, Logan V, Husselbee S, Zhou J, Law M. Recurrent urinary tract infections in women with symptoms of lower urinary tract dysfunction. Int Urogynecol J. 2009, 2:837-842.
- Dietz HP Quantification of major morphological abnormalities of the levator ani. Ultrasound Obstet Gynecol. 2007, 29:329-334.






Leave a Reply