The pharmacology of combined oral contraceptives old and new.
Access to contraceptive choice is important for women, and their partners, to control the number and spacing of their children. Contraceptives can be classified by their duration of action. Despite the increasing recognition that the long-acting reversible contraception (LARC) methods, namely implants, intrauterine methods and depot injections, offer highly effective and cost-effective pregnancy prevention across the reproductive lifespan, the combined oral contraceptive pill (COC) remains the most commonly used method by Australian women. Reasons for the relatively high use of the COC and low use of LARC, compared to similar developed countries appear to include lack of awareness and misconceptions by women and their healthcare providers, habitual prescribing by GPs and an ability to stop and start the COC without medical intervention.1 Additional non-contraceptive benefits of combined oral contraceptives, including acne control and an ability to manipulate bleeding patterns, can be attractive attributes for some women.2
This article focuses on the pharmacology of the various combined oral contraceptive pills available on the Australian market, with specific reference to their side-effect and risk profile.
The first combined oral contraceptives, marketed in 1961, contained relatively high doses of oestrogen and progestogen hormones and were associated with a relatively high risk of venous thromboembolic (VTE) and arterial disease and a high likelihood of unwanted side-effects, such as nausea and breast tenderness. The risks have been reduced by the development of combined oral contraceptives with lower doses of oestrogen and an awareness of safe prescribing. Safe prescribing, taking a woman’s risk factors into account, remains paramount. Guidelines for the safe provision of contraception include the World Health Organisation (WHO) Medical Eligibility Criteria (MEC)3 and UK-based MEC guidelines from the Faculty of Sexual and Reproductive Health (FSRH).4,5
Developments in COC formulations
The earliest contraceptive pill was a progestogen-only formulation at a dose that effectively inhibited ovulation although, in common with today’s progestogen-only contraceptives, it was associated with unpredictable ‘breakthrough’ bleeding resulting from an unstable endometrium. Historically, the accidental contamination of some pill batches with oestrogen resulted in improved ‘cycle control’ as well as enhanced contraceptive effectiveness – and as a result the ‘combined pill’ was born.2 The original COC contained norethynodrel, a derivative of 19-nor testosterone, and 150μg of mestranol, a prodrug of ethinyl oestradiol (EE).2
Newer progestogens were subsequently developed in an attempt to minimise androgenic and other troublesome side-effects while the dose of EE was reduced to potentially lower the VTE risk and oestrogenic side-effects such as nausea and breast tenderness. All currently available combined oral contraceptives have an effect on metabolic parameters, including oestrogen-related increases in sex hormone-binding globulin (SHBG), corticosteroid-binding globulin (CBG) angiotensinogen and apolipoprotein A1 and a change in various coagulation and fibrinolysis factors, which are modulated by the combination and dose of oestrogen and progestogen in a given pill type.6 It is important to note, however, that the effects of hormonal combinations on metabolic markers for vascular disease do not always directly translate into disease outcomes and controversy continues about the modulating effect of progestogen type on VTE risk. The dose effect of EE on VTE risk is clearer however with today’s low dose COCs, containing 35μg of EE or less, posing a low VTE risk when appropriately prescribed. Since 2010, COCs containing either 17 β oestradial or its prodrug oestradiol valerate in place of EE have been available. These oestrogens are structurally identical to the 17 β oestradiol (E2) produced by the ovary. In the laboratory setting, COCs with 17 β oestradiol/oestradiol valerate perform favourably compared with EE pills in relation to their effect on coagulation factors and insulin resistance. However, we will need to wait some years until the completion of post-marketing surveillance studies on these newer pill formulations to see if these effects translate into improved health outcomes.2
In relation to the COC progestogen component, earlier laboratory and registry studies suggested that pills with levonorgestrel (LNG) are associated with a lower VTE risk than those containing newer progestogens. However, later large, well-designed cohort studies have not confirmed a difference in risk between progestogen types7 and a recent controlled, prospective, observational, active surveillance study of more than 85 000 women that compared a COC containing 30μg EE and drospirenone in an extended 24- day regimen to other commonly used COCs in a routine clinical setting, supports a lack of difference in the risk of serious adverse cardiovascular events between available COC types.8 While all large database studies can be criticised for bias7, it is reasonable to conclude that a woman’s own underlying risk factors for VTE or arterial vascular disease have a much more significant impact on her risk than any differences between product constituents.6
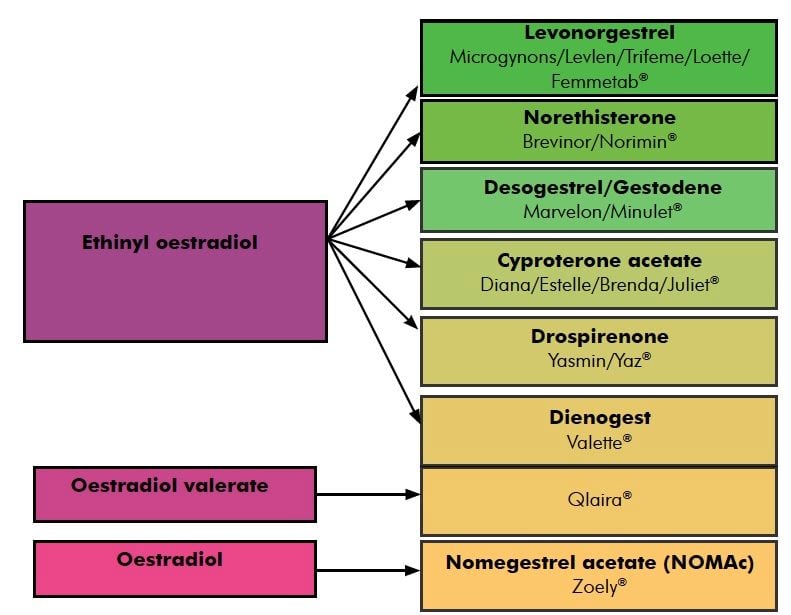
Figure 1. COCP types in Australia (not all generics are included).
The oestrogen component of combined oral contraceptives
Figure 1 shows the constituents of most of the different combined oral contraceptives available in Australia. The majority contain the potent synthetic steroid EE which, as a result of its 17α ethinyl group, has a pronounced effect on hepatic metabolism. Today’s low-dose COCs contain 35μg or less of EE. It is unknown whether the lowest dose pill formulations available in Australia (with 20μg EE) offer a safety benefit over those with 35 or 30μg EE and any potential safety advantage must be offset by an increased chance of unpredictable breakthrough bleeding.5
Administering EE via a non-oral route, as a vaginal ring or patch (not currently available in Australia), does not appear to mitigate its hepatic impact6 with one study suggesting an increased risk of VTE for non-oral delivery methods.9 Earlier attempts to substitute EE with oestradiol, in an effort to potentially reduce cardiovascular risk, had been thwarted owing to unfavourable bleeding patterns, but this has been successfully overcome by novel combinations with either desogestrel (Qlaira) or nomogestrol acetate (Zoely) thanks to their strong anti-proliferative endometrial effect.10 The 17β-oestrodiol/nomogestrel acetate pill is monophasic (in other words all pills contain the same hormonal dose) with a four-day hormone-free break while the oestradiol valerate/desogestrel combination is quadriphasic with an increasing oestrogen and decreasing progestogen dose through the pill pack. While all COCs reduce menstrual blood loss and can be useful for the management of heavy menstrual bleeding (HMB), both the 17β-oestrodiol and oestradiol valerate pills are associated with a high chance of an absent withdrawal bleed in the pill-free break. Qlaira has an additional indication for the management of HMB in women requiring contraception.
In addition to their effect on blood loss, 17β-oestrodiol and its prodrug oestrodiol valerate, are associated with less pronounced effects on haemostatic and lipid variables.6 Whereas EE increases VLDL levels, 17β-oestradiol increases the HDL level without increasing the VLDL level.6 While the effects on laboratory-based vascular disease markers are favourable for these newer COCs, evidence for their effect on VTE and arterial vascular disease is pending.
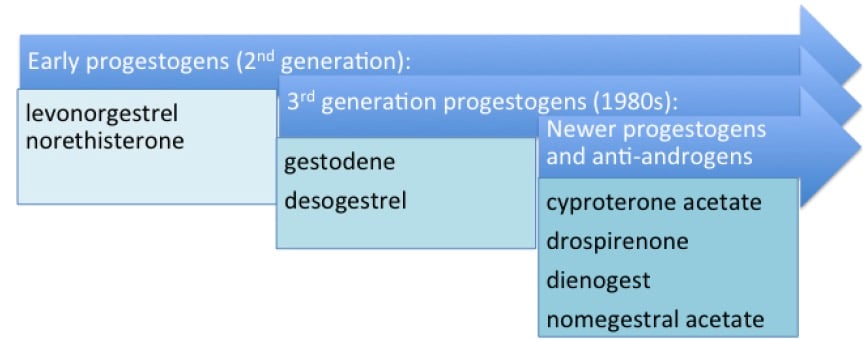
Figure 2. The development of progestogens.
The progestogen component of combined oral contraceptives
While progesterone itself cannot be used in the COC owing to its rapid liver metabolism, the synthetic progestogens in combined oral contraceptives are structurally related either to progesterone (pregnanes and 19-norpregnanes) or to testosterone (T) (estranes and gonanes), see Table 1. Several newer progestogens have been developed to bind more selectively to the progesterone receptor while minimising androgenic, oestrogenic and/or glucocorticoid receptor interactions and their related potential side-effects.6 The earliest progestogens, LNG and norethisterone (NET) are derived from testosterone. while the later progestogens have been derived from progesterone or its related mineralocorticoid, spironolactone (see Figure 2).
Table 1. Pharmacologic classification of progestogens used in contraceptives (available contraceptives and contraceptive agents in development)6.
| Related to progesterone | Related to testosterone |
|---|---|
Pure progestational (19nor-pregnanes)
|
Partly estrogenic and androgenic (estranes)
|
Antiandrogenic
|
Partly androgenic (gonanes)
|
Partly glucocorticoid
|
Anti-androgenic (non-ethyl estrane)
|
Related to spironolactone antialdosterone and antiandrogenic
|
Progestogens and vascular risk
Progestogen-only contraceptives including the progestogen-only pills, the contraceptive implant and LNG-releasing IUD, do not appear to increase the risk of VTE. Despite a growing body of evidence pointing to a minimal role of progestogen type in the COC on VTE risk compared to oestrogen, this topic remains controversial.7 Recurrent media scares about the pill and VTE risk create confusion and anxiety for women, so it is essential for doctors to provide clear balanced evidence-based information when prescribing contraception. The risk of VTE for women using any of the low-dose COCs (pills containing 35μg or less of EE) appears to be approximately two-to-six fold2 compared to nonusers which is substantially lower than the risk associated with pregnancy or the postnatal period.
Despite the lack of strong evidence for a clinically relevant difference between progestogens, it is postulated that the more androgenic progestogens, such as LNG and NET, are able to counteract the potent EE-induced stimulation of liver proteins and coagulation factors, while non- or anti-androgenic progestogens such as drospirenone have a limited mitigating effect on EE’s action.6 The COCs which contain the anti-androgen cyproterone acetate have also been associated with an increased risk of VTE compared to LNG COCs11, although this information is based on relatively small case-control studies.
The most androgenic progestogens may be associated with impaired glucose tolerance and increased insulin resistance, both risk factors for cardiovascular disease and Type II diabetes mellitus.6 Again, evidence for clinically relevant differences between progestogen types is lacking and the use of COCs in women with polycystic ovarian syndrome (PCOS) has not, for example, been found to be associated with clinically significant adverse metabolic consequences.12
In contrast to VTE risk, the delivery of oestrogen and progestogen vaginally via the EE/etonogestrel vaginal ring does not appear to affect insulin sensitivity.6 Progestogen-only pills, the contraceptive implant and LNG-releasing IUD have a minimal effect on lipids.6
In practice, we can conclude that women with pre-existing risk factors for venous or arterial disease, which make the COC an unsafe option, still have progestogen-only or non-hormonal highly effective methods to choose from (see Table 2). Conversely, women with no contraindications to the use of oestrogen can potentially use any of the available COCs with choice being determined by side-effects, additional non-contraceptive benefits, cost and personal preference.
Table 2 MEC* categories with risk factors for VTE (not including the first six weeks postpartum).5
| Condition | CHCs | POPs ENGimplants,DMPA, LNGIUD | Cu-IUD | LNG-EC | |
|---|---|---|---|---|---|
| Past history of VTE | 4 | 2 | 1 | 1 | |
| Current VTE, on anticoagulant | 4 | 2 | 1 | 2 | |
| Family history of VTE | First degree relative aged <45 | 3 | 1 | 1 | 1 |
| First degree relative aged ≥45 years | 2 | 1 | 1 | 1 | |
| Known thrombogenic mutation | 4 | 2 | 1 | 1 | |
| BMI 30-34kg/m2 | 2 | 1 | 1 | 1 | |
| BMI ≥35kg/m2 | 3 | 1 | 1 | 1 | |
| Surgery with prolonged immobilisation | 4 | 2 | 1 | 1 | |
| Surgery without prolonged immobilisation | 2 | 1 | 1 | 1 | |
| Immobilisation unrelated to surgery | 3 | 1 | 1 | 1 | |
| Thrombophlebitis | 2 | 1 | 1 | 1 | |
*MEC 1: a condition for which there is no restriction for the use of the contraceptive method
MEC 2: a condition where the advantages of using the method generally outweigh the theoretical or proven risks
MEC 3: a condition where the theoretical or proven risks usually outweigh the advantages of using the method
MEC 4: a condition which represents an unacceptable health risk if the contraceptive method is used
Side-effects and non-contraceptive benefits
Drosperinone, dienogest and nomegestrol acetate are newer progestogens designed to bind more specifically to the progesterone receptor and to minimise side effects related to androgenic, oestrogenic or glucocorticoid receptor interactions experienced with the earlier progestogens.6 Drosperinone, a derivative of spironolactone, counteracts the effect of EE-induced increases in angiotensin and aldosterone production, which lead to water and salt retention.13 It also has anti-androgenic properties. Dienogest is a non-ethyl estrane progestogen that has no androgenic action, but rather an anti-androgenic effect.14 Nomegestrol acetate (NOMAc) is a derivative of 19-norprogesterone, with a strong anti-proliferative effect on the endometrium, producing good cycle control combined with some anti-androgenic properties.15 In a randomised, open label, comparative trial evaluating effects on haemostasis, lipids and carbohydrate metabolism, NOMAc 2.5mg/17β oestradiol (E2) 1.5mg showed less impact overall on lipid metabolism as well as other parameters than a COC containing LNG 150μg/EE 30μg per daily pill.16
Cyproterone acetate, an anti-androgen with weak progestogenic activity, in combination with EE provides highly effective treatment for hyperandrogenism as well as providing effective contraception. Combined oral contraceptives with anti-androgenic progestogens (drospirenone or dienogest) or less androgenic progestogens (gestodene, desogestrel or NOMAc) also have a theoretical advantage for women who request treatment for androgenic symptoms. However, it is important to be aware that the oestrogenic component of all COCs is likely to improve acne via increased SHBG levels and a reduction of free testosterone, even at a low dose and even when combined with an androgenic progestogen. A Cochrane review concluded that few important differences were found between COC types in their effectiveness for treating acne.5
In practice, the evidence for the benefit of one COC type over another is often limited in relation to the management of troublesome side effects or for additional non-contraceptive benefits on acne and menstrual bleeding and it may be a matter of trial and error in finding the most appropriate formulation to suit an individual woman.
Conclusion
While increasing awareness and uptake of LARC methods are likely to impact on the proportion of women using combined oral contraceptives in the future, many women will continue to choose an oral method of fertility control. There have been significant changes to COC formulations over the past 50 years, including the development of progestogens with anti-androgenic activity and positive benefits on HMB, and the replacement of EE with oestradiol. However, future developments in hormonal contraception are more likely to be related to alternative delivery systems, especially those which can be either self-administered or minimise the woman’s need to interact with the health system.
In the meantime, it remains imperative that combined oral contraceptives are prescribed safely according to the WHO/FSRH Medical Eligibility Criteria. Since differences between COC types as a result of their progestogen and oestrogenic constituents appear to have little impact on relative safety, COC choice for eligible women will therefore be based on individual preference in relation to potential side-effect profile, additional non-contraceptive benefits as well as cost. While the earlier LNG and NET COCs are subsidised by the PBS in Australia, subsidy of the newer formulations would require a body of evidence demonstrating clinical and/or economic superiority over the older established pill types. We await with interest the outcomes of post-marketing surveillance studies of the newer COC formulations.
Acknowledgement
The authors wish to thank Amanda Young, Family Planning NSW acting librarian, for assistance with referencing.
References
- Kirsten I Black, Deborah Bateson and Caroline Harvey. Australian women need increased access to long-acting reversible contraception. Med J Aust 2013; 199 (5): 317-318.
- Paula Briggs, Gabor Kovacs, John Guillebaud (editors) Contraception: a casebook from menarche to menopause. Cambridge: Cambridge University Press, 2013.
- World Health Organization. Medical eligibility criteria for contraceptive use. 4th ed. [Internet] Geneva: WHO; 2009 [cited 2014 April 17]. Available from: http://whqlibdoc.who.int/ publications/2010/9789241563888_eng.pdf .
- Faculty of Family Planning and Reproductive Healthcare. UK medical eligibility criteria for contraceptive use. [Internet] London: FSRH; 2009 [cited 2014 April 17]. Available from: www.fsrh.org/pdfs/ UKMEC2009.pdf .
- Bateson D, Harvey C, McNamee K. Contraception: an Australian clinical practice handbook. 3rd ed. Brisbane 2012.
- Sitruk-Ware R, Nath A. Metabolic effects of contraceptive steroids. Rev Endocr Metab Disord. 2011; 12:63–75. Doi: 10.1007/s11154-011- 9182-4.
- Bitzer J. Position statement. Statement on combined hormonal contraceptives containing third- or fourth-generation progestogens or cyproterone acetate, and the associated risk of thromboembolism. Eur J Contracept Reprod Health Care. 2013; 18:143-147.
- Dinger J, Bardenheuer K, Heinemann K. Cardiovascular and general safety of a 24-day regimen of drospirenone-containing combined oral contraceptives: final results from the International Active Surveillance Study of Women Taking Oral Contraceptives. Contraception 2014 Apr; 89(4): 253-63. doi: 10.1016/j.contraception.2014.01.023. Epub 2014 Feb.
- Lidegaard O, Nielson L, Skovlund CW, Lokkegaard E. Venous thrombosis in users of non-oral hormonal contraception: follow-up study, Denmark 2001-10. BMJ 2012;344:e2990.
- Fruzzetti F, Trémollieres F, Bitzer J. An overview of the development of combined oral contraceptives containing estradiol: focus on estradiol valerate/dienogest. Gynecol Endocrinol 2012;28:400-8. Doi: 10.3109/09513590.2012.662547.
- Vasilakis-Scaramozza C, Jick H. Risk of venous thromboembolism with cyproterone or levonorgestrel contraceptives. Lancet. 2001;358(9291):1427-9.
- Halperin IJ, Kumar SS, Stroup DF, Laredo SE. The association between the combined oral contraceptive pill and insulin resistance, dysglycemia and dyslipidemia in women with poly-cystic ovary syndrome: a systematic review and meta-analysis of observational studies. Hum Reprod. 2011;26:191-201.
- Oelkers W, Foidart JM, Dombrovicz N, et al. Effects of a new oral contraceptive containing an antimineralocorticoid progestogen, drospirenone, on the renin-aldosterone system, body weight, blood pressure, glucose tolerance, and lipid metabolism. J Clin Endocrinol Metab. 1995;80:1816-21.
- Sasagawa S, Shimizu Y, Kami H, Takeuchi T, Mita S, Imada K, et.al. Dienogest is a selective progesterone receptor agonist in transactivation analysis with potent oral endometrial activity due to its efficient pharmacokinetic profile. Steroids. 2008;73:222-31.
- Ruana X, Seegerb H, Mueck AO. The pharmacology of nomegestrol acetate. Maturitas. 2012;71:345-53.
- Ågren UM, Anttila M, Mäenpää-Liukko K, Rantala ML, Rautiainen H, Sommer WF, Mommers E. Effects of a monophasic combined oral contraceptive containing nomegestrol acetate and 17β-oestradiol compared with one containing levonorgestrel and ethinylestradiol on haemostasis, lipids and carbohydrate metabolism. Eur J Contracept Reprod Health Care. 2011 Dec;16(6):444-57. Doi: 10.3109/13625187.2011.604450.








Leave a Reply