A review of the pharmacology of clinically proven treatments for the common symptoms of menopause.
The menopause, defined as the permanent cessation of ovarian follicular activity, or, in practical terms as the absence of menses for more than 12 months in a woman who has not undergone hysterectomy, normally occurs within a ten-year window from age 45–55, with a mean age of 51.5 years in the Australian population.
The menopause is associated with a variety of short- and long-term symptoms. The commonest acute symptoms are the vasomotor symptoms of hot flushes and night sweats, followed by joint and muscle pains, insomnia, vulval symptoms and poor memory. Figure 1 illustrates common menopausal symptoms with differences in incidence experienced around the world. Interestingly, these differences are seen within different national and ethnic subgroups as well. Long-term consequences of the menopause include osteoporosis, with increased risk of associated fracture, and cardiovascular disease. These symptoms have one common thread; they are owing to withdrawal of oestrogen in a previously oestrogen-primed woman.
Vasomotor symptoms are the most common presenting symptom in Western women. Core body temperature is controlled by a temperature control centre within the medial pre-optic area of the hypothalamus.1
In premenopausal women physiological levels of oestrogen maintain endorphin levels within the hypothalamus, but when oestrogen declines after the menopause there is a resulting release of noradrenergic activity from its tonic inhibition. This leads to increased hypothalamic release of noradrenaline and serotonin, leading to a lowering of the sweating threshold in the thermoregulatory centre. Post-menopausal women who experience hot flushes have a greater narrowing of the thermoregulatory zone than those women who are symptom free and are, therefore, more sensitive to small increases in core temperature.2 Studies have confirmed that oestrogen administration widens the thermoregulatory zone.3
Menopausal arthralgia has been reported in more than 50 per cent of Australian women, with an even greater percentage following surgical menopause and in Asian women. The same symptoms are commonly seen following the initiation of aromatase inhibitor therapy.4 Oestrogen withdrawal is clearly the cause and the mechanism of action appears to be related to the actions of sex hormones on pain processing pathways, immune cells and chondrocytes.
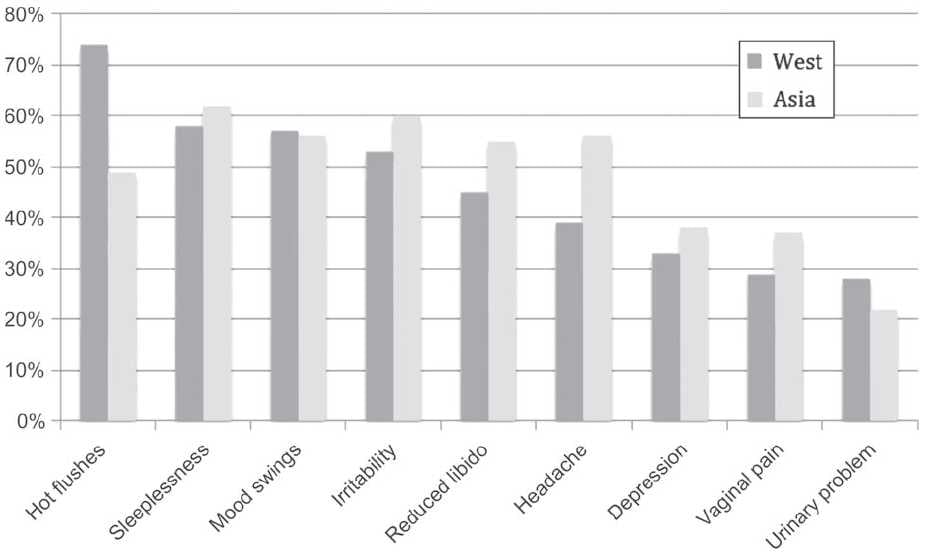
Figure 1. Prevalence of menopausal symptoms in Asian and European women. From Baber R East is east and West is west: perspectives on the menopause in Asia and The West. Climacteric 2014;17:23-28 (reproduced with permission).
Vulval symptoms are also very common and result from the effect of the hypo-oestrogenic state on the vulval and vaginal epithelium. These changes result in vulval and vaginal dryness and itchiness, as well as superficial dyspareunia and sexual dysfunction.
Oestrogen
Oestrogen withdrawal is at the heart of menopausal symptoms thus, not surprisingly, oestrogen replacement has proven to be the most effective treatment. Oestrogen exerts the majority of its effects in a traditional endocrine manner, via genomic [transcriptional] effects mediated by hormone binding to the oestrogen alpha and beta receptors.
The effect of oestradiol on vasomotor symptoms is genomically mediated, is dose dependent and has proven to be the most effective treatment of vasomotor symptoms.5 The effects of oestrogen on the cardiovascular systems are also largely genomic, however there are some non-genomic actions resulting from the recruitment of signalling pathways that are often associated with cell membrane receptors, ion channels or enzyme linked receptors. The complex interplay of transcriptional and non-transcriptional mechanisms results in increased production of nitric oxide, with resultant rapid vasodilatation of blood vessels, blocking the vessel wall’s response to injury and decreasing the development of atherosclerosis.6 Similarly, in studies of the effects of oestrogen on bone, while the major effects are mediated via ERs found on osteoclasts, non-transcriptional effects have also been found, resulting in a net response of increased bone density.
The route of administration of oestrogen may affect the side-effect profile, with increasing evidence that topical oestrogen patches are associated with a reduced risk of venous thromboembolic and cerebovascular events compared to oral oestrogen, probably owing to the former bypassing hepatic first-pass metabolism.7
Local oestrogen treatment of vulval symptoms is extremely effective, and there was no increase in incidence of endometrial hyperplasia when compared to placebo in a Cochrane meta-analysis.8 However, the rate of systemic absorption is variable and topical oestriol may be preferable to topical oestradiol, as the receptor binding affinity of oestriol is 80 times weaker than that of oestradiol.
Progestogens
Progestogens are a group of C21 carbon structures containing a pregnane skeleton that bind to progesterone receptors. They include naturally occurring progesterone and the synthetic progestins.
The principal role of progestogens is to protect the endometrium from oestrogenic stimulation, as unopposed oestrogenic stimulation of the endometrium will lead to endometrial hyperplasia, atypical hyperplasia and endometrial cancer. Numerous studies have shown that administration of a progestogen for ten to 14 days per month will induce secretory change in the endometrium and prevent hyperplastic change. This action is mediated genomically via the progesterone receptors PR a and PR b.
While natural progesterone and the retro progesterone dydrogesterone bind mainly to the progesterone receptors, the synthetic progestins not only bind to the progesterone receptor but also bind to other steroid receptors including glucocorticoid, mineralocorticoid, androgen and oestrogen receptors. This can give rise to unwanted side effects including the apparent increase in risk of breast cancer seen with long-term use of oestrogen combined with synthetic progestins compared to natural progesterone or dydrogesterone.9
Therefore, progestogens should only be used in women with an intact uterus or in women receiving HRT for debilitating menopausal symptoms shortly after surgical treatment of endometrial cancer.
Testosterone
Testosterone is produced in the adrenal cortex and the ovary. Levels decline following the menopause and are more pronounced following a surgical menopause. There is no level of testosterone below which a woman can be described as androgen deficient. However, several randomised clinical trials of testosterone in doses appropriate for women have shown significant benefit in the treatment of hypoactive sexual desire and arousal disorders.10 Testosterone exerts its primary effects genomically via testosterone receptors; however, it is important to note that aromatasealso converts testosterone to oestrogen. Therefore, without testosterone women cannot produce oestrogen and testosterone supplementation will inevitably result in some oestrogen production as well.
Tibolone
Tibolone is a synthetic compound exerting oestrogenic, progestogenic and androgenic properties. The parent compound has only weak activity, but is metabolised in the gut into two hydroxylated metabolites that exert their clinical effects primarily by binding to the oestrogen receptors. A Delta 4 isomer acts as a weak progestogen, exerting its effect via the progesterone receptors, and the parent molecule also exerts a weak androgenic effect via the androgen receptor. Tibolone and its metabolites also inhibit the action of a breast specific sulphatase enzyme and 17-beta hydroxy steroid dehydrogenase type 2, resulting in reduced oestrogenic stimulation of the breast. Thus although tibolone alleviates vasomotor symptoms, improves bone density and reduces fractures it does so via a combination of metabolic, enzyme-mediated and receptor-mediated actions.11
Non-hormonal treatments
SSRIs and SNRIs
Perhaps the most commonly used non-hormonal prescription treatments for hot flushes are the antidepressants, usually in doses much lower than those required to treat major depressive illness. Selective serotonin re-uptake inhibitors (SSRIs) are potent inhibitors of serotonin re-uptake. Serotonin noradrenaline re-uptake inhibitors (SNRIs) are potent inhibitors of serotonin and noradrenaline re-uptake, although in many cases low-dose SNRIs will block only serotonin re-uptake and high-dose SNRIs will block re-uptake of serotonin, noradrenaline and also dopaminergic neurotransmission. As mentioned earlier, the mechanism underlying hot flushes appears related to a disorder in thermoregulation triggered by oestrogen withdrawal and mediated by either adrenergic or serotonergic neurotransmission.12 Given that SSRIs inhibit only serotonin re-uptake and low-dose SNRIs act principally via the same mechanism, it seems most likely that alleviation of vasomotor symptoms is via the effect of the drugs on serotonin.
Perhaps the most widely used is venlafaxine in doses of 37.5–75mg. The active metabolite of venlafaxine, desvenlafaxine13-16, in doses of 50–100mg daily has also proved effective, as have escitalopram17 and citalopram.18 Unlike the treatment of depressive symptoms, alleviation of hot flushes with these treatments is usually rapid, within one or two weeks. Side effects are typical of all antidepressants, but usually mild because of the low doses used. Paroxetine has also been shown to be effective and was recently approved by the FDA in a very low dose of 7.5mg daily.
Some SSRI/SNRIs have the potential to interfere with tamoxifen metabolism. Paroxetine and fluoxetine are potent inhibitors of CYP2D6 in vitro, while venlafaxine, desvenlafaxine, citalopram and escitalopram have little or no effect. Therefore, the latter should be preferred in women receiving tamoxifen therapy. A randomised cross-over trial of gabapentin and venlafaxine showed a similar reduction in hot flushes, but women preferred venlafaxine.19
Clonidine
Clonidine stimulates alpha 2 adrenoreceptors in the brain stem resulting in reduced sympathetic outflow from the central nervous system and decreased peripheral resistance, heart rate and blood pressure.16 Normal postural reflexes are unaffected. Clonidine’s principal indication is to treat hypertension but it has been demonstrated to have a modest impact on vasomotor symptoms. The mechanism for this action remains uncertain, but may be owing to reduced catecholamine production or alternatively to reduced peripheral vasodilatation.
Doses used are usually 50µg twice daily and in those doses side effects are generally mild and include dry mouth, constipation, drowsiness and dizziness. As with most interventions, an improvement in symptomatology should be seen within four to six weeks.20
Gabapentin and pregabalin
Gabapentin and pregabalin are analogues of gamma aminobutyric acid (GABA) and were originally developed to treat epilepsy and neuropathic pain. Gabapentin, in particular, has also been shown to be effective, in a dose-dependent manner, in the treatment of vasomotor symptoms of the menopause.
The mechanisms of action of gabapentin and pregabalin remain unclear. Despite their origins, neither drug has activity in the GABAergic neurotransmitter system. Gabapentin increases the concentration and probably the rate of synthesis of GABA in the brain, it binds with high affinity to a novel binding site in the brain associated with voltage-sensitive calcium channels, it appears to modulate certain types of calcium current, it reduces the release of several monoamine neurotransmitters and it increases serotonin concentrations in human whole blood.
Not surprisingly, the mechanism of action of gabapentin in alleviating hot flushes has not been fully elucidated. Gabapentin inhibits neuronal calcium currents in vitro, binding to alpha 2 gamma voltage-gated calcium channels. This binding site is substantially up-regulated in response to peripheral nerve injury and may be similarly up-regulated in response to oestrogen withdrawal leading to increased activity of neurotransmitters in the hypothalamus.21
In randomised controlled trials, gabapentin in doses of 900mg per day has been shown to be significantly better than placebo in the treatment of hot flushes.22 Dizziness and somnolence are the commonest side effects and both are dose dependent as well as reversible.
Summary
Although oestrogen (in combination with progestogen for endometrial protection) is the most effective way of treating vasomotor symptoms, there are many non-hormonal options available. There is increasing evidence that the route of oestrogen administration may change the side-effect profile and topical oestrogen is an effective way of treating postmenopausal vulval symptoms.
References
- Casper R and Yen S. Neuroendocrinology of hot flushes. Clin Endocrinol. 1985;22:293-312.
- Freedman R and Krell W. Reduced thermoregulatory null zone in post menopausal women with hot flashes. Am J Obstet Gynecol. 1999;181:66-70.
- Freedman R. Physiology of hot flashes. Am J Hum Biol. 2001;13: 453-64.
- Magliano M. Menopausal Arthralgia. Menopause 2010;67:29-33.
- MacLennan AH. Cochrane database systematic reviews 2004 CD002978.
- Simoncini and Genazzani. Non genomic action of sex steroid hormones. European Journal of Endocrinology. 2003;148:281-92.
- Canonico M et al. Hormone therapy and venous thromboembolism among postmenopausal women: impact of the route of oestrogen administration and progestogens: the ESTHER study. Circulation 2007;115:84-5.
- Suckling J et al. Local oestrogen for vaginal atrophy in postmenopausal women. Cochrane Database of Systematic Reviews 2006 CD001500.
- Fournier A et al ‘Unequal risks for breast cancer associated with different hormone replacement therapies: results from the E3N Cohort study. Breast Cancer Res Treat. 2008;107:103-11.
- Somboonporn W, Bell R, Davis S. Testosterone for peri and postmenopausal women. Cochrane Database of Systematic Reviews 2005 CD004509.
- Kloosterboer H J. Tibolone: a steroid with a tissue specific mode of action. Steroid Biochem Mol Biol. 2001;76:231-8.
- Evans M et al Management of hot flushes with venlafaxine: A RCT. Obstet Gynecol. 2005;105:161-6.
- Bouchard P et al Randomized placebo- and active- controlled study of desvenlafaxine for menopausal vasomotor symptoms. Climacteric 2012;15:12-20.
- Pinkerton J et al. Maintenance of the efficacy of desvenlafaxine in menopausal vasomotor symptoms: a 1-year randomized controlled trial. Menopause. 2013;20:38-46.
- Pinkerton et al. Desvenlafaxine compared with placebo for treatment of menopausal vasomotor symptoms: a 12-week, multicenter, parallelgroup, randomized, double-blind, placebo-controlled efficacy trial. Menopause. 2013;20:28-37.
- Nelson H et al. Nonhormonal therapies for menopausal hot flashes: systematic review and meta-analysis. JAMA. 2006;295:2057-71
- Freedman R, Kruger M, Tancer M. Ecitalopram treatment of menopausal hot flashes. Menopause. 2011;18:893-6.
- Barton D et al. Phase III, placebo-controlled trial of three doses of citalopram for the treatment of hot flashes. J Clin Oncol. 2010;28:3278-83.
- Bordeleau L et al. Multicenter, randomized, cross-over clinical trial of venlafaxine versus gabapentin for the management of hot flashes in breast cancer survivors. J Clin Oncol. 2010;28:5147-52.
- Neill M. Clinical pharmacology of Clonidine. Curr Clin Pharmacol 2011;6:280-7.
- Pandya et al. Gabapentin for hot flashes in 420 women with breast cancer: a randomised controlled trial. Lancet. 2005;366:818-824.
- Guttuso T Jr et al. Gabapentin’s effects on hot flashes in postmenopausal women: a randomized controlled trial. Obstet Gynecol. 2003;101:337-45







Leave a Reply