Prostaglandins are a diverse group of substances we use therapeutically on a daily basis. In other circumstances we use drugs to block their effects. An understanding of their biochemistry, physiology and pharmacology will only enhance our obstetrics, gynaecology and general practices.
Hardly a day passes in our practices where we don’t utter the word prostaglandin, use prostaglandins (PG) therapeutically or block their effect with drugs. The term ‘prostaglandin’ was coined in 1935, before anything was known of their structure. In 1961 there was one published report on them in the medical literature, by 1973 there were over 1000 and today Google comes up with 1,280,000 entries in 0.10 seconds.
Yet what do we know about these mysterious substances? After all, many of us would never have received a single lecture about them in medical school. What are they; where are they and how do they exert their effects? This article will hope to shed light on this for you.
History
In 1930, two New York gynaecologists Kurzrok and Lieb, while studying the effects of human semen on strips of human uterus (as you do), noticed that the muscle contracted and relaxed. Later, in the mid-1930s, Goldblatt, an Englishman, and Ulf von Euler from Sweden independently reported the smooth muscle contracting effects of sheep seminal fluid. Von Euler named this lipid-soluble acid ‘prostaglandin’ for obvious reasons.
Years passed, but in 1964 Bergstrom and von Dorp, also independently, were able to synthesise prostaglandin E2 (PGE2), the active ingredient of Prostin, from arachidonic acid (AA). By 1971 it was discovered that aspirin and related drugs inhibited prostaglandin synthesis. In 1975, 1976 and 1983, thromboxane A2, prostacyclin (PGI2) and leukotrienes were respectively discovered.
The term ‘prostaglandin’ has turned out to be a misnomer as these substances are found in small amounts in all human tissues except erythrocytes. Their effects are protean and often paradoxical.
Synthesis and biochemistry
PG are also known as eicosanoids, in other words autocoids, from eicosatrienoic acid (dihomoλlinoleic acid), which is an essential 20 carbon omega 2 fatty acid. All prostaglandins are 20 carbon molecules. The linoleic acid is converted enzymatically to AA (see Figure 1) , the father of all prostaglandins, thromboxanes and leukotrienes.
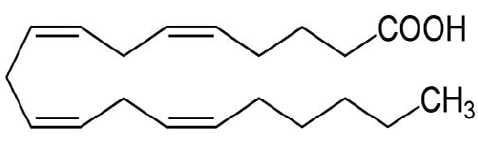
Figure 1. The chemical structure of arachidonic acid.
Once synthesised, AA is stored by esterification to the phospholipids of cell membranes and other complex lipids.
The concentration of free AA in cells is very low. The biosynthesis of eicosanoids is dependent upon the availability of this important precursor. It has to be released from the cell membrane for use either by the enzyme phospholipase A2 or an influx of calcium ions. These are released in response to physical, chemical and hormonal stimuli.
Once released, AA is metabolised rapidly (see Figure 2) to oxygenated products, including prostaglandins, by a ubiquitous complex of microsomal enzymes such as cycloxygenases (COX 1 and 2) and lipoxygenases. Essentially, there are two pathways. AA is metabolised to PGs and thromboxanes by COXs and to leukotrienes by lipoxygenases. Both prostaglandins and leukotrienes are proinflammatory and released in times of stress.
COX 1 and 2 initially convert AA to PGG2 (prostaglandin G2). This rapidly morphs non-enzymatically into the chemically unstable PGH2, which has three main metabolic pathways depending on the specific tissues and their requirements at the time. Firstly, it can be converted to the obstetrically famous PGE2 by an isomerase enzyme or by a reductase to PGF2α, also familiar to all readers.
Endothelial cells are rich in prostacyclin synthase and this enzyme converts PGH2 to PGI2 (also known as prostacyclin) which is a potent vasodilator and inhibitor of platelet aggregation. Like all prostaglandins it has a typically short half life of three minutes. In direct juxtaposition to this are platelets, which have abundant thromboxane synthase. This enzyme converts PGH2 to thromboxane A2 (t1/2 30 seconds), a very potent vasoconstrictor and platelet aggregator. All of this is important in order to be able to understand non-steroidal anti-inflammatory drugs (NSAIDs), their function and controversies.
The leukotrienes are proinflammatory and produced in most white cell types as well as alveolar cells and are important in the pathophysiology of asthma. A leukotriene receptor blocker called montelukast (Singulair) is used as an asthma prophylactic, especially in children. These important substances will not be discussed further in this article.
Physiology of prostaglandins
Prostaglandins sustain homeostatic function within cells and mediate pathogenic mechanisms including the inflammatory response. They are not endocrine hormones, they are autocrine or paracrine. In other words, they are produced locally and either act on the cells they are produced by (autocrine) or surrounding cells (paracrine). The interesting thing about prostaglandins is, unlike most enzymes, they are not proteins, but lipids.
Their effects are mediated by a number of distinct receptors, all with specific prostaglandin agonists. Ten receptors have been described to date. Their nomenclature is not relevant to daily clinical practice.
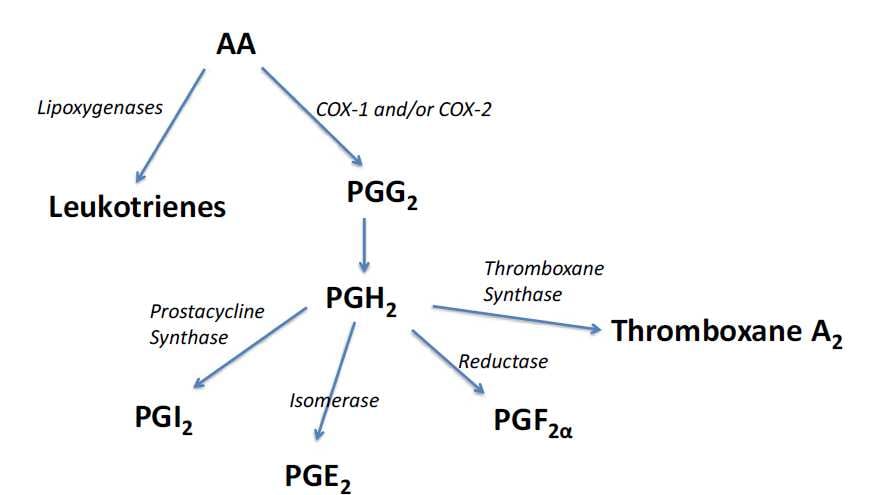
Figure 2. Conversion pathways for the common prostaglandins.
Stimulation of these receptors either results in facilitation or inhibition of adenyl cyclase and hence the concentration of cyclic AMP and the relevant protein and enzyme production.
They are metabolised and their effect terminated by prostaglandin dehydrogenase on first pass through the lungs hence their super short half lives. Their metabolic effects are diverse and dependent upon which prostaglandin and which tissue is involved. Their effects on platelets and vascular smooth muscle have already been alluded to. Table 1 summarises the effects of the more important prostaglandins to our specialty.
An important effect of prostaglandins is their action on the stomach. They are able to protect the stomach lining by a number of mechanisms including the inhibition of gastric acid secretion and an increase in gastric mucus production. Hence the prostaglandin analogue misoprostol (cytotec) relieves ulcer pain and promotes ulcer healing with an efficacy approaching that of omeprazole. Unfortunately, the side effect of diarrhoea limits its usefulness, but it has a niche where it is used in people who have to take NSAIDs despite a predisposition to peptic ulcer disease.
Effect of prostaglandins on reproduction and parturition
In obstetric and gynaecology practice, the effect and use of prostaglandins are integral. Firstly, it is thought they may have an important role in fertility, especially with regards to fertilisation. Their high concentration in semen and efficient vaginal absorption is suggestive of this with demonstrable effects on the uterus, fallopian tubes and sperm transport.
With regards to menstruation, it has been found that there are high concentrations of prostaglandins in the menstrual fluid. They have a role in causing uterine contractions, increased gastrointestinal peristalsis and sensitisation of afferent pain fibres. All these factors contribute to dysmenorrhoea. It is for this reason that NSAIDs (COX inhibitors) work well for period pain and are more effective than narcotic-based analgesics.
| Prostaglandin type | Major sites of synthesis | Major biological activities |
|---|---|---|
| PGD2 | Mast cells, eosinophils, brain | Inflammation Bronchoconstriction |
| PGE1 | Most tissues | Vasodilation Platelet aggregation |
| PGE2 | Kidney, spleen, heart, uterus | Vasodilation Platelet aggregation Uterine contraction Maintains patency of ductus ateriosus |
| PGF2α | Kidney, spleen, heart | Vasoconstriction Bronchoconstriction Uterine contraction |
| PGI2 (Prostacyclin) | Heart, endothelial cells | Platelet and white cell aggregation Vasodilation |
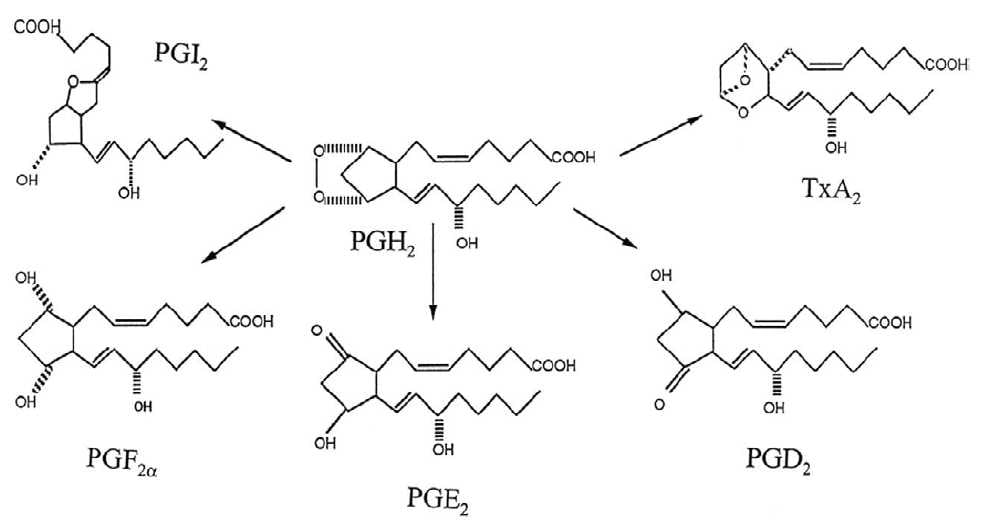
Figure 3. Chemical structures of the common prostaglandins.
During pregnancy, the ability of the fetal membranes to elaborate prostaglandins rises progressively and during labour the concentration of prostaglandins in the blood and the amniotic fluid is elevated. The significance of this is uncertain as is how much the onset of labour is determined by prostaglandins. Of all the prostaglandins, the E and F series result in uterine contractions with E being much more uterine selective and certainly more superior in cervical ripening. One of the therapeutic advantages of prostaglandins is that prostaglandin receptors are always present in the myometrium whereas oxytocin receptors develop during pregnancy, not peaking till the last part of pregnancy and even during labour. This allows prostaglandins to be used earlier in pregnancy for instance in induction of labour for fetal death in the second trimester when oxytocins would never work.
Therapeutics
Because prostaglandins affect all tissues, their therapeutic uses are diverse. Blocking their action is also therapeutic as they cause inflammation that is often pathological, such as in osteoarthritis. As we saw earlier, they are also useful in period pain.
Options
Agonism
- Induction of labour – PGE2 (Dinoprostone or Prostin), misoprostol.
- Therapeutic termination of pregnancy – PGF2α (Dinoprost),
misoprostol (usually with mifepristone). - Treatment of postpartum haemorrhage (PPH) – misprostol,
intramyometrial prostaglandin F2α. - Management of miscarriages (including missed miscarriages) –
misoprostol. - Maintenance of patency in cases of patent ductus arteriosus
(PDA) – PGE1 (Prostin VR). - Impotence – PGE1 (Caverject).
- Gastric cytoprotection – misoprostol (Cytotec).
- Treatment of glaucoma – latanoprost (Xalatan).
Antagonism
NSAIDs – non-selective COX inhibitors, for example, indomethacin, naproxen, diclofenac, ibuprofen and aspirin; or selective COX 2 inhibitors, for example, celecoxib and meloxicam.
Selected examples relevant to O and G
Agonism
- Prostaglandin E2 chemical name dinoprostone (trade names Prostin, Cervidil): this is used in medical inductions to ripen the cervix in an attempt to get the patient into labour. It is inserted as an intra-vaginal gel (Prostin 1 or 2mg) or as a 10mg pessary attached to a tape (Cervidil). It acts by dissociating collagen fibrils and altering the mucopolysaccharide composition of the cervix. It promotes cervical ripening by softening and effacing. It has a pharmacological half life of less than one minute and its primary metabolite less than ten minutes, though this is half as potent as the original PGE2. It promotes a more natural labour than artificial rupture of the membranes and oxytocin.
- Misoprostol (Cytotec): this drug is a prostaglandin E1 analogue and comes as a 200µg tablet. Originally developed for peptic ulcer treatment, it is now used off label for a variety of indications in obstetrics and gynaecology. These include medical abortion, management of miscarriage, induction of labour, cervical ripening before medical procedures and the treatment of postpartum haemorrhage (PPH). It has a number of advantages. It is low in cost, doesn’t need refrigeration, has a long shelf life and can be used orally as well as rectally, vaginally, sublingually and buccally.
- Gemeprost (trade name Cervagem): this drug is a PG analogue, probably 10–200 times more potent than PGE1, PGE2 or PGF2α. It comes as a 1mg pessary. Its main effect is to act locally with minimal systemic absorption to soften and dilate the cervix. It can be used prior to intrauterine operative procedures in the first trimester of pregnancy, but is mainly used for therapeutic termination in the second trimester of pregnancy, often for death in utero.
- Prostin F2α injection (trade name Dinoprost): this comes in a 5m/ml injection. It used to be used extra-amniotically for mid trimester termination, but has been surpassed by newer drugs. It is now used mainly as an intra-myometrial injection for severe treatment-resistant PPH.
Antagonism
NSAIDs act by blocking, either reversibly or irreversibly, COXs, which are the first enzymes in the prostaglandin synthetic pathway. There are two: COX 1 and COX 2. COX 1 is the constitutive form of the enzyme found everywhere in most tissues such as blood vessels, stomach, uterus and kidney. COX 2, however, is induced in settings of inflammation by cytokines and other inflammatory mediators. This enzyme is inhibited by corticosteroids as well as NSAIDs and, more particularly, the COX 2 inhibitors.
All NSAIDs have analgesic, anti-pyretic, anti-inflammatory and to a less extent anti-platelet properties. They reversibly bind to COX enzymes and their action lasts as long as the half life of the drug. Most of them are non-selective.
Aspirin was the original drug in this group and is slightly unique in its action. It irreversibly binds to both COX 1 and 2. It covalently acetylates the enzymes by binding to serine. Its effect is as long as the enzyme lasts in that particular tissue. In platelets this is about seven to ten days. That is why patients need to be off aspirin for this length of time before surgery.
The ideal dose of aspirin as an anti-platelet agent is uncertain, but is thought to be about 160mg. If this dose is exceeded by too much it will block prostacyclin production as well as thromboxane synthesis hence losing its antithrombotic effect.
As COX 2 is only produced in sites of inflammation, blocking this enzyme selectively is advantageous. It is able to block the inflammatory effects without blocking the important functions such as gastric protection. That is the theoretical advantage of drugs like celecoxib (Celebrex). One downside is that COX 2 is a major source of PGI2 (prostacyclin) production so blocking this enzyme may have some theoretical prothrombotic tendency. This may have been a contributing factor to Vioxx being taken off the market.
References
Further reading
Joel G. Hardman and Lee E. Limbird (Editors). Goodman and Gillman’s The Pharmacological Basis of Therapeutics McGraw Hill Tenth Edition 2001 Chapter 26. This article is largely based on information from this text. Rebecca Allen and Barbara M O’Brien. Uses of Misoprostol in Obstetrics and Gynaecology. Reviews in Obstetrics and Gynaecology. 2009 Summer, 2(3):159-168.
Michael W King. Introduction to Eicosanoids themedicalbiochemistrypage. org accessed Dec 2013.
Donald E Wilson. The Medical Significance of Prostaglandins. Archives of Internal Medicine. Vol 133, Jan 1974 p29.






Leave a Reply