An overview of screening and diagnostic breast imaging in Australia.
There are standard guidelines for population screening for breast cancer. Since its introduction, ‘BreastScreen Australia has had a major impact in moderating an increasing incidence trend and in contributing to falling mortality in breast cancer.’ (Australian Institute of Health and Welfare report, 2014).
With the decline in mortality, thought to be thanks to a combination of early detection from the national screening program and continued improvements in treatment, breast cancer is now considered a chronic disease rather than one of mortality.
Incidence and risk factors
Breast cancer is the most common type of cancer in Australian women, with an incidence of 27 per cent. One in eight Australian women will be diagnosed with breast cancer by the time they turn 85. The average age at diagnosis is 62 years old. Approximately 75 per cent of the women diagnosed are over the age of 50; of the remainder, the majority are aged between 40 and 50 years old. Breast Cancer Network Australia (BCNA) estimated that in 2014, 15 270 women would be diagnosed with breast cancer, which translates to approximately 42 women being diagnosed each day in 2014.
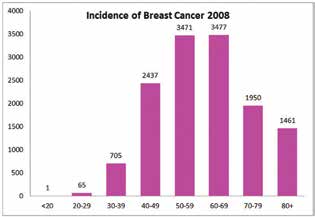
Figure 1. The incidence of breast cancer in 2008.
Known risk factors include the following: increasing age, family history, obesity, alcohol consumption, hormone-replacement therapy, no children or children after 35, no breastfeeding, early onset menarche and late onset menopause.
Recommendations for screening
Currently, the recommended national guidelines in Australia are for biennial screening 2D mammogram for asymptomatic women in the target age group of 50–74 years old (recently expanded to 74 years old, previous invitation to attend screening included 50–69 year olds).
The age at which a woman should commence with screening will depend upon her risk factors. Typically, it is considered appropriate to commence five to ten years before the age of diagnosis of a first-degree relative with breast cancer, or 50 years old, whichever is earliest. However, as 25 per cent of breast cancers occur in the under-50 age group, many women may wish to consider biennial screening from 40 years old.
‘Breast cancer is the most common type of cancer in Australian women…One in eight Australian women will be diagnosed with breast cancer by the time they turn 85.’
The benefit of screening in this younger age group is somewhat contentious; as it is believed that biennial screening is not frequent enough given the tumour biology is typically of higher grade and thus faster growing for the two-year time interval to be of benefit.
In the UK, screening is offered from 45 years old and this is currently under consideration in Australia. The current guidelines in Australia are that a woman may attend the national BreastScreen program between 40 and 49 years old or beyond 74 years old; however, this is by patient request rather than direct invitation.
Many women lie outside the low-risk group, either owing to family history of breast or ovarian cancer, personal risk factors such as a past history of atypia on biopsy, personal history of breast cancer or having mammographic dense BIRADS 4(D) breasts.
Depending upon these factors, a woman will be classified as being at either moderate or strong risk and, accordingly, may require annual rather than biennial screening mammograms, annual breast magnetic resonance imaging (MRI) and annual clinical review with a breast examination by a breast surgeon or breast physician.
Diagnostic mammography
Women with symptoms such as a lump, nipple discharge, skin changes or new symptoms should be assessed with a diagnostic mammogram +/- supplemental ultrasound and not in a population screening program.
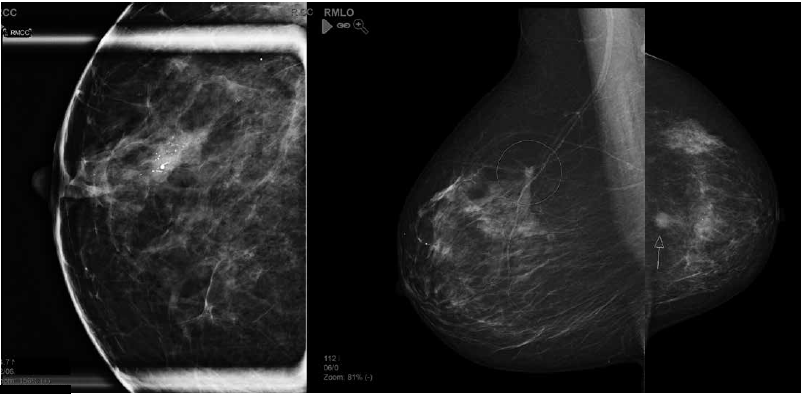
Figure 2. Examples of mammographic abnormalities. Left to right: a. casting calcifications – high-grade ductal carcinoma in situ; b. stellate – carcinoma; c.mass – carcinoma.
The distinguishing factor between screening and diagnostic imaging is that standard 4 MMG views are taken in screening. Whereas if a patient has a symptom then diagnostic views will often involve additional spot or localised compression to the area of concern and assessment in person by a radiologist, including other imaging such as tomosynthesis, ultrasound or recommendation for MRI. Symptomatic woman are therefore assessed outside of screening programs, either in the private radiology firms, breast clinics or public hospital breast centres.
Mammographic density classification
Research by Boyd has shown that there is an association between mammographic density and breast cancer risk. Based upon the American College of Radiology Mammographic BIRADS 5 Atlas grading score of ‘A, B, C, D’, where ‘A’ is fatty replaced breast tissue and ‘D’ is extremely dense, the latter is associated with an increased risk of breast cancer of four-to-six fold. This replaces the previous classification of BIRADS 1,2,3,4, which referred to quartile percentages of mammographic density.
This is a dilemma for standard mammography because, as the risk of cancer increases with mammographic density, the sensitivity of detecting the cancers on the mammogram decreases, since the cancers are hidden or camouflaged by the dense breast tissue that surrounds them. With the knowledge of risk association with mammographic density, and as an indicator of sensitivity of mammography, there is increasingly more demand for the patient and referring doctor to be informed of the mammographic density.
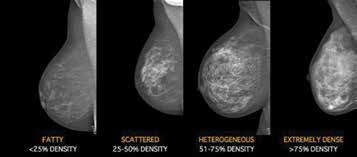
Figure 3. Representative Mammogram examples of BIRADS mammography
density: BIRADS A – the breasts are almost entirely fatty; BIRADS B – there
are scattered areas of fibroglandular density; BIRADS C – the breasts are
heterogeneously dense, which may obscure small masses; BIRADS D – the
breasts are extremely dense, which lowers the sensitivity of mammography.
Mammographic density can be assessed by subjective means, based upon the 2D mammogram, or by computer software programs as a volumetric measurement of per cent of breast density. The computer software programs assess the density using 3D imaging, which is automatically generated by the software program once the mammogram images have been taken. The ramifications of mammographic density are evolving and guidelines are, as yet, not available on when and what supplemental screening should be instituted. The available options are ultrasound, tomosynthesis and MRI, but the availability is dependant on the breast service, whether it is in the private or public system. The national screening programs do not currently have tomosynthesis or MRI.
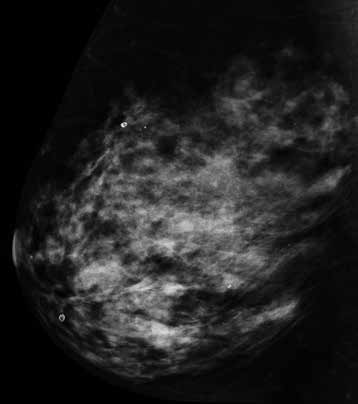
Figure 4a. Example of BIRADS D mammography density, no abnormality perceived on standard 2D mammogram or ultrasound. BIRADS D – The breasts are extremely dense, which lowers the sensitivity of mammography.
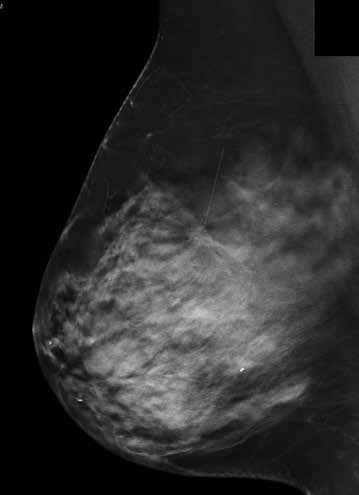
Figure 4b. Tomosynthesis MLO image of breast shown in 4a) reveals small stellate lesion with architectural distortion.
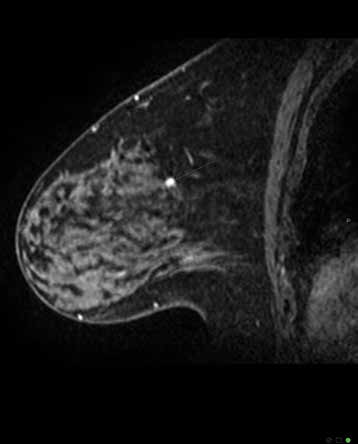
Figure 4c. MRI Magnetic Resonance Imaging sagittal view of breast shown in 4a. and b. confirms highly suspicious lesion – histopathology 12mm invasive ductal carcinoma grade 2.
Digital breast tomosynthesis
Standard 2D mammography has inherent limitations. It displays a 3D volume of breast tissue as a 2D image, and so two orthogonal views of each breast are standard practice. Despite this, standard 2D mammography has limitations with composite or summation shadow effects, and this is most pronounced in mammographic dense breasts.
The limitation of mammography is owing to the minimal differences in soft tissue densities between the components and structures that make up breast tissue. Cancers are typically of similar density and thus mammography relies on identifying subtle differences in density and architectural distortion in order to detect the cancer.
With standard 2D mammography, it is not uncommon to require additional spot coned compression views in an attempt to determine if the apparent or real mammographic density is owing to a superimposition/summation shadow effect or indeed a real lesion, such as a cancer. The result of the compression view is to reduce overlying tissue effects and accentuate the contrast of the breast components.
Digital breast tomosynthesis (DBT) is an advanced application of digital technology. It has been developed to attempt to overcome some of the limitations of standard 2D digital mammography. Following the results of numerous trials, the implementation of tomosynthesis is gaining momentum, as a potential replacement of standard 2D mammography for screening as well as assessment of breast tissue. DBT involves a series of low dose x-rays of the breast, obtained at varied angles, creating a data set of images and a 3D mammogram. The benefit of DBT is the reduction in the overlapping tissue, superimposition and masking effect of traditional mammography.
Studies have shown that DBT:
- increases cancer detection;
- reduces recall rates for superimposition of breast tissue;
- reduces false positive rates;
- reduces the need for extra spot compression mammography;
- reduces the need for ultrasound.
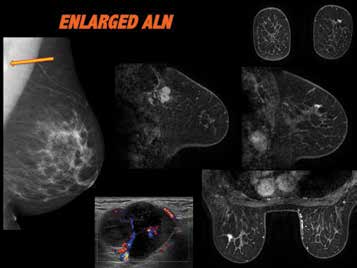
Figure 5. BIRADS B mammographic density, standard 2D MMG reveals
abnormal left axillary lymph node, which appears of matted nodal mass
configuration on ultrasound. Malignant on fine needle biopsy, suggestive
of breast primary origin. No breast abnormality perceived on mammogram
or ultrasound. MRI reveals small stellate left breast upper outer quadrant,
confirmed as malignant on biopsy. Outcome: 17mm invasive lobular
carcinoma grade 2 & LCIS, ER/PR +, 20/20 LN positive.
Breast MRI
MRI is used widely for screening high-risk women and for the staging of breast cancer. The risk for breast cancer is variable and complex and, as such, some patients with certain risk factors may benefit from this form of supplemental screening. The most common group to benefit from screening MRI is women with a strong family history and gene carriers. Breast MRI is the most sensitive test available for detecting breast cancer. It does not, however, replace a mammogram, but rather is a supplement to it. The main advantage of MRI is that it is independent of breast density, unlike standard 2D mammography where the sensitivity of cancer detection reduces as mammographic density increases. It does not use ionising radiation, nor involve compression of the breast as in 2D mammography and DBT.
MRI uses principles of high spatial and temporal resolution and tumour angiogenesis. High spatial resolution allows detection of small cancers <5mm and improved characterisation of lesion morphology. To achieve this a strong magnet and high channel coil are required. High temporal resolution allows more accurate lesion kinetics, which is the evaluation of the tumour angiogenesis. Cancers release angiogenesis factor that promotes the growth of new blood vessels around tumour. These vessels are abnormal, demonstrate arterio-venous shunting and are ‘leaky vessels’, which show a kinetics pattern of rapid enhancement following gadolinium contrast injection.
One disadvantage of MRI is the time taken to perform the study is typically 25 minutes and requires the injection of intravenous contrast (gadolinium).
MRI is available for breast cancer screening to all women who are eligible under the Medicare guidelines and may be referred by a specialist. For those women who are not rebatable and who may still benefit from an MRI, the study may be referred by a general practitioner or specialist, as appropriate, and will be an out-of-pocket expense for the patient.
There are a number of indications where MRI may be considered that are currently not covered by the guidelines. These include: staging of breast cancer; mammographic dense breasts; moderate risk factors – family history (those not eligible for rebate), past atypia on biopsy, lobular carcinoma in situ; malignant axillary node with normal mammogram; neo-adjuvant therapy response; and unexplained symptoms.
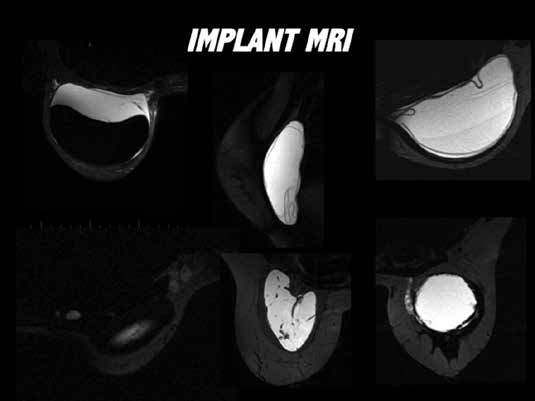
Figure 6. Examples of application of MRI evaluation of implants: a. top left
– peri-implant space abacteraemic collection, implant intact; b. middle top
– fully collapsed intra capsular rupture, ‘linguine sign’; c. top right – dual gel
silicone, minimally collapsed intracapsular rupture; d. bottom left – silicone
adenopathy in axillary and internal mammary chain nodes; e. middle bottom
– Poly Implant Prosthesis rupture, ‘fracture pattern’; f. bottom right – extra
capsular rupture.
Implant MRI
Many women have breast augmentation, either for cosmetic or reconstructive reasons. Ultrasound assessment of implant integrity is significantly limited by the ability of the sound waves to penetrate the anatomical boundaries that surround the implant and the consistency of the implant gel. Implant MRI is the gold standard in evaluating implant integrity. It allows for assessment of rupture, gel bleed phenomenon or rejection response. It is not a cancer screening study and does not involve intravenous injection.
Further reading
Boyd, N et al. Mammographic Density and the Risk and Detection of Breast Cancer: N Engl J Med. 2007;356:227-236.
Skaane et al. Comparison of Digital Mammography Alone and Digital Mammography Plus Tomosynthesis in a Population-based Screening Program. Radiology. Volume 267: Number 1 April 2013.
Houssami, N; STORM, a new dimension for mammography screening: Evidence of improved detection using integrated 2D and 3D mammography provides opportunity for Australia to lead screening trials. MJA. 2013;199(5)2. Lockie, D et al; Evaluation of Digital Breast Tomosynthesis (DBT) in a Breast Screen assessment service. Journal of Medical Radiation Sciences. Volume 61, Issue Supplement S1, pages 63–112, Sept 2014.
Breast MRI Medicare Guidelines in Australia: www.health.gov.au/internet/main/publishing.nsf/Content/pathol-di-mri-breastmriqa .






Leave a Reply