Imaging safely, particularly in pregnancy – what to consider.
The decision to refer any patient for imaging is always a balance between the probability that the imaging test will improve management and the risks associated with the test, including the risks of radiation. With pregnant or potentially pregnant patients the risks associated with ionising radiation are a major consideration.
There are two main types of risks for exposure to ionising radiation:
- Short-term (deterministic) risk: this is from cell death and may result in skin erythema, cataracts and hair loss. This is dose dependent, in other words, both the risk and the severity of the injury increase as the radiation dose increases. Deterministic effects are rarely seen in normal diagnostic imaging as substantial radiation dose thresholds must be exceeded, but they might result after numerous CT and PET/CT scans or after lengthy interventional procedures.
- Long-term (stochastic) risk: this is owing to DNA damage and may result in radiation-induced cancer. Although this risk increases with dose, there is no threshold to be exceeded as injury to a single cell or group of cells may be sufficient. This risk is expressed as chances per million and is derived from data collected from survivors of the atomic bombs in Japan and from others exposed to high radiation doses. It is only an estimate. The severity of the stochastic effect is independent of the radiation dose.
Pregnancy
A woman who is pregnant, is herself no more sensitive to radiation than a woman who is not pregnant. The risk to the fetus from ionising radiation depends on:
- the part of the mother’s body exposed to the radiation;
- the stage of pregnancy; and
- the radiation dose received.
Short-term risk to the fetus
The short-term risk is different for a fetus compared with a child or adult because it is rapidly growing. The risks include death, slowing of normal growth, abnormal growth and being intellectually or emotionally underdeveloped. The fetus is most vulnerable to radiation-induced congenital malformations during the period of organogenesis, that is weeks three to eight after conception. From weeks eight to 15 the fetus has the highest risk of radiation-induced neurodevelopmental abnormalities.
The International Commission on Radiation Protection (ICRP) has stated that these deterministic risks are not expected to occur if the exposure is <100mGy of radiation.1 Table 1 provides the expected dose for a fetus with different examinations. Even the highest dose examinations are well below 100mGy. Multiple examinations or multiphase scans are of more concern.
Long-term risk to the fetus
It was first demonstrated in 1956 that there is an increased incidence of leukaemia and childhood cancer in children born to mothers who received diagnostic pelvic irradiation in pregnancy.2 The association between prenatal radiation exposure and childhood cancer is now well recognised.
Table 1 shows the additional risk of developing cancer over and above the background risk (1:500 children develop cancer [risk from birth to 14 years] with no exposure to radiation as a fetus). Even if the mother had the highest dose examination the additional risk of cancer might increase three fold, in other words, the absolute risk is still low. If a high dose examination is needed for the mother and the risk of not having it is significant then it is appropriate to perform it.
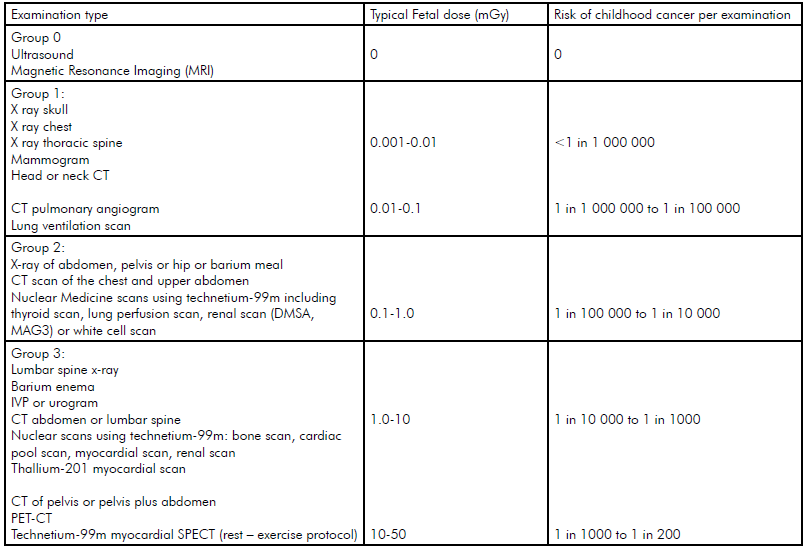
Table 1. Typical fetal doses and risks of childhood cancer for common radiology. Note: Natural Childhood risk of cancer is 1 in 500. Advice from the UK Health Protection Agency, the Royal College of Radiologists and the College of Radiographers.
General comments
The fundamental principle in imaging any patient is to keep the radiation dose to nil, or as low as reasonably achievable (ALARA). Many clinical questions can be answered with ultrasound or magnetic resonance imaging (MRI), neither of which use ionising radiation.
There is no evidence that greyscale ultrasound has any adverse effects.3 4 The thermal effect of pulsed Doppler ultrasound is of concern in embryos and fetuses and the British Medical Ultrasound Society has rigorous recommendations about the safety (T1 thermal and M1 mechanical) indices on machines.5 For this reason, pulsed Doppler imaging should be used prudently generally in pregnancy and especially in early pregnancy.
MRI radiofrequency fields generate heat, but there is no evidence of adverse effects in pregnancy, although study samples are small.6 It is unknown if higher magnet strengths (>1.5 Tesla) and prolonged imaging times may have biological effects at sensitive stages of development so it is recommended to perform fetal MRI after 18 weeks gestation whenever possible and only when the clinical benefits outweigh the potential risks.7
It is crucial that any woman referred for imaging is asked if she could be pregnant and this is particularly important in nuclear imaging or if the abdomen or pelvis will be exposed to radiation. If a woman is not sexually active it is safe to proceed with these tests. If a woman is sexually active and her period is not overdue it is considered safe to proceed with lower dose procedures (see Groups 1–3 in Table 1).8
If a woman is sexually active and is referred for a procedure that involves more than 10mGy to the uterus it is extremely important to be sure she is not pregnant and, unless it is an emergency, the examination should be delayed until the first ten days of the next menstrual cycle or until her Beta human chorionic gonadotropin (BHGC) is confirmed to be negative before proceeding. If the woman is pregnant then the potential risk of not having the test needs to be balanced against the potential risk to the fetus.
This will require close collaboration between the referring clinician and the radiologist. As mentioned, radiation risks are most significant during organogenesis: the first trimester. The central nervous system (CNS) is highly sensitive to radiation from eight to 15 weeks post-conception and doses of 100mGy may result in a decreased IQ. The CNS only becomes less sensitive to radiation after 25 weeks gestation.9 10
Very occasionally, a patient has an abdominal/pelvic computed tomography (CT) scan before realising she is pregnant. As organogenesis starts three-to-five weeks post-conception, the risk of malformation is not thought to be increased and the main risk is death of the conceptus and then only if the dose is more than 100mGy.11 12 Even if the dose is less than 100mGy, it is prudent to have a medical physicist calculate the estimated dose. Unless the embryo is older than three weeks it is likely the mother can be reassured that the effect on her baby will have been minimal.
If a test using ionising radiation is essential in pregnancy then, wherever possible, dose reduction techniques – such as lowering the miliamperes or increasing the pitch with spiral CT – are important to minimise the dose to the fetus. Diagnostic studies remote from the fetus, for example, limb or chest, can be done safely at any stage of pregnancy (assuming the equipment is in proper working order). Shielding the abdomen and pelvis should be used if these areas are not being imaged. Mammography is similarly considered safe at all stages of pregnancy.13
Is there a pulmonary embolus?
The question of how to exclude a pulmonary embolus (PE) in a pregnant patient is a highly complex one and there is no complete agreement on the best imaging pathway.14 15 16 17 18 19 It is generally agreed that a Doppler ultrasound of the legs is the first step.20 21 22 If positive, the mother can be treated for the venous thrombosis/presumed PE. If negative and a chest X ray is clear, then the difficult question is whether to do a ventilation-perfusion (VQ) scan or CT pulmonary angiogram (CTPA). There is no clear consensus on this. A VQ scan will give a higher radiation dose to the fetus (see Table 212) although the difference is less marked in the third trimester. A CTPA scan has a higher dose to the mother and this is of particular concern in younger women in view of the stochastic risk to the breasts, which are more radiation sensitive. In addition a CTPA scan in pregnancy is more likely to be inadequate (six to 36 per cent), owing to the hyperdynamic circulation and limited breath holding. Repeating a CTPA scan should be only done with caution for the reasons already mentioned. There are no known mutagenic or teratogenic effects from intravenous iodinated contrast media.23
Table 2. Radiation dose for mother and fetus.24 Note: Background dose is 2.5mSv per year
Note: Background dose is 2.5mSv per year
Other important factors to consider are the local availability of nuclear medicine studies, the age of the equipment in the medical imaging department, the rate of technically inadequate studies in the local department and, perhaps most importantly, the degree of clinical suspicion (anecdotally only two-to-four per cent of CTPAs performed are positive for a pulmonary embolus). The decision of which imaging pathway to take is therefore best made after close consultation with the local radiologist. Some departments recommend a VQ scan first, others a CTPA scan. The imaging pathway from the Western Australian Government’s Diagnostic Imaging Pathways25 suggests a CTPA first in the first and second trimesters and VQ scan first in the third trimester. MR angiograms are in their infancy and at this stage are available and have not been established as a definitive test.26 27
Other imaging matters
MRI is the modality of choice for staging cancer in pregnant patients although using gadolinium is not recommended as the long-term risk of gadolinium to the fetus is not yet known.28 29 If a woman is breastfeeding, the radiopharmaceutical from a nuclear medicine study will be distributed throughout her body and some will enter the breastmilk. To avoid exposing the infant to radiation, is it best to stop the breastfeeding for a short period after the examination. Again, close communication with the nuclear medicine specialist will determine if this is necessary and if so for how long.
Conclusion
The decision to refer a pregnant woman to radiology should be made after considering the risks of the test to both mother and fetus versus the risks of not doing the test. The potential of the test to alter management is central and consultation with a radiologist can be helpful in determining the best course of action for the patient and in ensuring that any radiation dose is as low as reasonably achievable.
References
- ICRP, 2000. Pregnancy and Medical Radiation. ICRP Publication 84. Ann. ICRP 30 (1).
- Giles D, Hewitt D, Stewart A, Webb J. Malignant disease in childhood and diagnostic irradiation in utero. Lancet. 1956 Sep 1;271(6940):447-447.
- Abramowicz JS. Benefits and risks of ultrasound in pregnancy. Semin Perinatol. 2013 Oct;37(5):295-300.
- BMUS (British Medical Ultrasound Society): Guidelines for the safe use of diagnostic ultrasound equipment. London: BMUS; 2009.
- BMUS (British Medical Ultrasound Society): Guidelines for the safe use of diagnostic ultrasound equipment. London: BMUS; 2009.
- Bulas D, Egloff A. Benefits and risks of MRI in pregnancy. Semin Perinatol. 2013 Oct;37(5):301-4.
- Bulas D, Egloff A. Benefits and risks of MRI in pregnancy. Semin Perinatol. 2013 Oct;37(5):301-4.
- Insideradiology.com.au internet Sydney: Inside Radiology – Radiation risk of medical imaging during pregnancy. updated 2011 Jan 31; cited 2014 Dec 15 www.insideradiology.com.au/pages/view.php?T_ id=96&ref_info#.VHZ2MdKUfHU .
- Nguyen CP, Goodman LH. Fetal risk in diagnostic radiology. Semin Ultrasound CT MR. 2012 Feb;33(1):4-10.
- iaea.org internet Vienna Austria. IAEA: Pregnancy and radiation protection in diagnostic radiology. updated 2013; cited 2014 Dec 15. Available from: https://rpop.iaea.org/RPOP/RPoP/Content/SpecialGroups/1_PregnantWomen/Pregnancyandradiology.htm .
- Nguyen CP, Goodman LH. Fetal risk in diagnostic radiology. Semin Ultrasound CT MR. 2012 Feb;33(1):4-10.
- iaea.org internet Vienna Austria. IAEA: Pregnancy and radiation protection in diagnostic radiology. updated 2013; cited 2014 Dec 15. Available from: https://rpop.iaea.org/RPOP/RPoP/Content/SpecialGroups/1_PregnantWomen/Pregnancyandradiology.htm .
- Nguyen CP, Goodman LH. Fetal risk in diagnostic radiology. Semin Ultrasound CT MR. 2012 Feb;33(1):4-10.
- imagingpathways.health.wa.gov.au internet Perth: Diagnostic Imaging Pathways– Obstetric and Gynaecological Pathways updated 2013 Oct; cited 2014 Dec 15 Available from: www. imagingpathways.health.wa.gov.au/index.php/imaging-pathways/obstetric-gynaecological .
- Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM, Hurwitz LM et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline–Evaluation of suspected pulmonary embolism in pregnancy. Radiology. 2012 Feb;262(2):635-46.
- Sadro CT, Dubinsky TJ. CT in Pregnancy: Risks and Benefits. Applied Radiology. 2013 Oct;42(10):6-16.
- Hunsaker AR, Lu MT, Goldhaber SZ, Rybicki FJ. Imaging in acute pulmonary embolism with special clinical scenarios. Circ Cardiovasc Imaging. 2010(Jul); 3(4):491-500.
- Bourjeily G, Paidas M, Khalil H, Rosene-Montella K, Rodger M. Pulmonary embolism in pregnancy. Lancet. 2010;375:500-12.
- Scarsbrook AF, Evans AL, Owen AR, Gleeson FV. Diagnosis of suspected venous thromboembolic disease in pregnancy. Clin Radiol. 2006;61: 1-12.
- imagingpathways.health.wa.gov.au internet Perth: Diagnostic Imaging Pathways– Obstetric and Gynaecological Pathways updated 2013 Oct; cited 2014 Dec 15 Available from: www. imagingpathways.health.wa.gov.au/index.php/imaging-pathways/obstetric-gynaecological .
- Sadro CT, Dubinsky TJ. CT in Pregnancy: Risks and Benefits. Applied Radiology. 2013 Oct;42(10):6-16.
- Scarsbrook AF, Evans AL, Owen AR, Gleeson FV. Diagnosis of suspected venous thromboembolic disease in pregnancy. Clin Radiol. 2006;61: 1-12.
- Sadro CT, Dubinsky TJ. CT in Pregnancy: Risks and Benefits. Applied Radiology. 2013 Oct;42(10):6-16.
- Hunsaker AR, Lu MT, Goldhaber SZ, Rybicki FJ. Imaging in acute pulmonary embolism with special clinical scenarios. Circ Cardiovasc Imaging. 2010(Jul); 3(4):491-500.
- imagingpathways.health.wa.gov.au internet Perth: Diagnostic Imaging Pathways– Obstetric and Gynaecological Pathways updated 2013 Oct; cited 2014 Dec 15 Available from: www. imagingpathways.health.wa.gov.au/index.php/imaging-pathways/obstetric-gynaecological .
- Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM, Hurwitz LM et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline–Evaluation of suspected pulmonary embolism in pregnancy. Radiology. 2012 Feb;262(2):635-46.
- Scarsbrook AF, Evans AL, Owen AR, Gleeson FV. Diagnosis of suspected venous thromboembolic disease in pregnancy. Clin Radiol. 2006;61: 1-12.
- Bulas D, Egloff A. Benefits and risks of MRI in pregnancy. Semin Perinatol. 2013 Oct;37(5):301-4.
- Leung AN, Bull TM, Jaeschke R, Lockwood CJ, Boiselle PM, Hurwitz LM et al. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline–Evaluation of suspected pulmonary embolism in pregnancy. Radiology. 2012 Feb;262(2):635-46.






Leave a Reply