The use of ultrasound imaging in the third trimester.
This article provides a brief overview of the use of ultrasound in the third trimester, with reference to the recently updated New Zealand MFM Network guideline for the management of suspected small for gestational age (SGA) singleton pregnancies and infants after 34 weeks gestation.1
Ultrasound
Ultrasound is the most commonly used imaging modality for assessing pregnancy. Although used as a diagnostic modality as early as the 1940s, the technology to acquire two-dimensional fetal images in real time was not available until the late 1970s.2 Rapid improvements in technology have led to real-time imaging, superior image quality, Doppler evaluation of blood flow and visualisation of the baby in three dimensions.
Dating
Determining gestational age based on biometric measurements assumes the size of the fetus is consistent with its gestational age. Biological variation increases with gestation, therefore the accuracy of dating scans decline as gestation advances; from approximately three-to-eight days in the first trimester to up to 35 days in the third trimester. When calculating gestational age >24 weeks, a combination of measures rather than a single biometric measurement is superior and a repeat scan should be considered for assessment of interval growth (at a minimum of two weeks).
Anatomy and anomaly screening
Screening for fetal anomalies is an important component of routine antenatal care. Standard screening is carried out at 18–20 weeks, allowing timely detection of serious and lethal abnormalities and maximising detection rates.3 Scanning for fetal anomalies may be required in the third trimester for women who present late in pregnancy; where anomalies are suspected or detected earlier, but the prognosis cannot be fully evaluated until the third trimester; or for conditions that may deteriorate with advancing gestation.
Growth
The majority of third-trimester ultrasound scans assess fetal growth, aiming to identify babies that are SGA (birthweight or estimated fetal weight [EFW] <10th centile) and/or have intrauterine growth restriction (IUGR), a fetus failing to reach its full growth potential. SGA and IUGR are strongly associated with an increased risk of perinatal mortality, with 40–50 per cent of normally formed stillborn babies having a birthweight <10th centile.4 Within a population of more than 92 000 singleton pregnancies, SGA was the largest population-attributable risk for stillbirth (22 per cent). Risk of stillbirth was five-fold greater when SGA was undetected antenatally compared to cases identified before birth (32 per cent versus six per cent).4 Identifying vulnerable babies in a timely fashion and ensuring appropriate management, including timely delivery, is crucial to reducing stillbirth.
However, routine third-trimester ultrasound scanning in an unselected population has not been shown to reduce perinatal mortality or morbidity: in a meta-analysis involving more than 27 000 pregnancies in eight randomised controlled trials, there was no difference in antenatal, obstetric or neonatal interventions or morbidity between screened and control groups.5 Routine scanning is therefore not currently recommended; however, this is a debatable issue and further trials are awaited in which scans are performed later in the third trimester at a time when the risk of growth concerns is higher. Women with significant risk factors for SGA should be considered for serial growth scans in the third trimester (see Table 1).
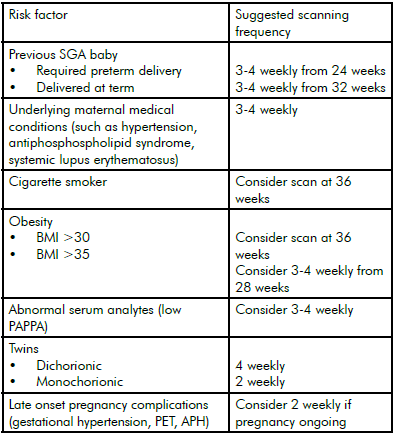
Table 1. Indications for routine growth scans in third trimester and suggested scanning frequency.
Recommendations adapted from NZMFM SGA guideline.6 Scanning may be required from earlier gestation or more frequently if additional risks are present and should be individualised for each case. Scanning should continue to time of delivery.
All other women should have symphysis-fundal height measured and plotted on an individualised gestation-related optimum weight (GROW) chart7 at each antenatal visit to identify others requiring growth scans. The use of GROW has been associated with a significant reduction in the number of second- and third-trimester scans required and an increase in antenatal detection of SGA babies from 29 per cent to 48 per cent.8
The standard measurements taken to assess fetal growth are biparietal diameter, head circumference, abdominal circumference (AC) and femur length (see Figure 1a–c). A variety of formulae exist that calculate EFW using a combination of these parameters. In a comparison of 35 different formulae, 29 produced a mean absolute percentage error ≤10 per cent: no one formula can be recommended as clearly superior.9 In Australasia, for babies weighing >1000g, the most widely accepted and used is the Hadlock C formula.10
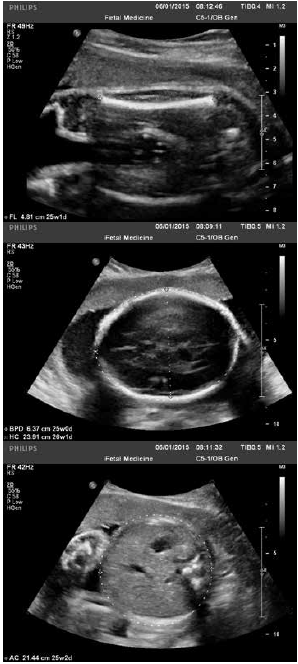
Figure 1. Standard biometry measures taken to assess fetal growth, from top: 1a. fetal femur length; 1b. fetal head measures, biparietal diameter and head circumference; 1c. fetal abdominal circumference.
All measurements should be plotted on centile charts. Individual growth parameters should be plotted on population-based ultrasound charts (such as ASUM charts)11 12 13 and the EFW plotted on GROW charts, which use a standard, longitudinal, ultrasound-derived curve of intrauterine weight gain and adjusts for maternal variables of ethnicity, parity, height and weight.14 The AC measurement is the most sensitive for detecting SGA and the measurement whose growth trajectory is likely to taper first. However, a number of scenarios should alert the clinician to suboptimal growth (see Table 2). All babies that are suspected to have suboptimal growth require umbilical artery (UmA) Doppler performed at the time of growth scan and, where resources permit, uterine artery (UtA) and fetal middle cerebral artery (MCA) Doppler studies should also be considered to further stratify risk (see below). Once fetal growth concerns have been identified serial scans should continue every two-to-three weeks until delivery if UmA Doppler remains normal.
Although babies that are large for gestational age (LGA) are at risk of increased morbidity and mortality, unlike SGA pregnancies, the antenatal detection of LGA pregnancies is not associated with improved maternal or perinatal outcomes. Serial scanning in a LGA pregnancy is not recommended to improve outcomes.15
Doppler ultrasound
Doppler ultrasound provides an assessment of blood velocity in fetal and maternal vascular territories. Doppler studies aid the assessment and management of pregnancy complications – notably SGA – but also other conditions, such as Rhesus isoimmunisation. Comparisons of flow velocity between systole and diastole provide valuable information on resistance to flow to the placenta (UmA or UtA) or redistribution of fetal circulation in response to hypoxia (MCA or ductus venosus). Systolic/diastolic ratio, resistance index (RI = systolic-diastolic/systolic flow) or pulsatility index (PI = [systolic-diastolic]/mean flow) are the main measures of resistance that may be reported. PI is the most commonly used and allows consideration of absent/reversed flow in diastole.
Umbilical artery
In cases of SGA, a reduction in diastolic relative to systolic flow is associated with adverse perinatal outcome, with absent and reversed Table 2. Ultrasound findings suggestive of suboptimal fetal growth.1 end diastolic flow associated with a four- and ten-fold increase in perinatal mortality, respectively.16 There is consistent evidence that monitoring the UmA Doppler in high-risk pregnancy reduces perinatal morbidity and mortality and antenatal admissions. As such, monitoring the UmA Doppler in SGA pregnancies should be the accepted clinical standard.17 18
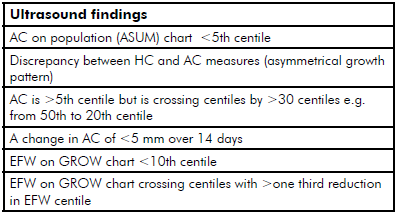
Table 2. Ultrasound findings suggestive of suboptimal fetal growth.19
Stratifying risk in the SGA baby
While the sequence of UmA Doppler waveform deterioration with progressive hypoxia is well documented in early-onset SGA, in most cases of late-onset SGA the UmA Doppler will remain normal. In these cases, perinatal morbidity is reduced compared to babies with an abnormal UmA Doppler, although these pregnancies may still suffer adverse outcomes attributable to SGA.20 21 22 It is recommended that SGA babies with normal UmA Doppler are further assessed with MCA and UtA Doppler to determine if they are at higher risk of adverse outcome.23
Uterine artery
In the third trimester UtA Doppler is a useful adjunctive prognostic indicator. In SGA pregnancies where the UtA waveform is abnormal (in other words, increased impedance to flow indicated by an elevated PI and/or bilateral diastolic notching is present), the baby is at greater risk of preterm delivery and lower birthweight and even where the UmA is normal, the UtA Doppler predicts an increased risk of emergency caesarean delivery.24
Fetal middle cerebral artery
In chronic hypoxia fetal blood is redistributed to the brain, heart and adrenals: a compensatory attempt to minimise hypoxic damage to these vital organs. Redistribution of fetal blood to the brain occurs via a relative vasodilation of the cerebral vasculature and is detected as a fall in RI or PI in the MCA Doppler. In late onset SGA, a fall in the MCA Doppler PI <fifth centile occurs in up to 15 per cent of cases and predicts risk of adverse outcome independent of UmA Doppler.25 The cerebro-placental ratio (CPR) is calculated as the ratio of UmA to MCA Doppler resistance. As the CPR is influenced by both rising UmA and falling MCA resistance, it becomes abnormal before either the MCA or UmA Doppler, and may be abnormal even if these individual components are within the normal range.26
Evaluation of the MCA Doppler is also an important component in the evaluation of fetal anaemia. Increasing anaemia results in reduced blood viscosity and redistribution of blood flow in favour
of the brain, resulting in increased peak systolic flow in the MCA. The use of the MCA Doppler to accurately assess fetal anaemia has revolutionised the management of Rhesus isoimmunisation, which previously required amniocentesis or cordocentesis.27
Fetal wellbeing
Amniotic fluid volume
In the third trimester, the main determinant of amniotic fluid volume (AFV) is the balance between fetal swallowing and urination. Abnormalities of AFV should prompt a review of the fetal urinary and gastrointestinal systems, as well as consideration of premature rupture of membranes and abnormal fetal growth. Qualitative or semi-quantitative (amniotic fluid index [AFI], single deepest vertical pocket [SDVP]) measurements are commonly used. AFI has less inter-observer variability than SDVP, but results in an increased diagnosis of oligohydramnios and subsequent intervention with no demonstrable improvement in perinatal outcome.28
Biophysical profile
The biophysical profile (BPP) assesses four ultrasound parameters (AFV, fetal breathing, tone and gross movements) in addition to a CTG recording. Evidence is lacking that the BPP is more effective at preventing perinatal mortality than conventional monitoring.29 The usefulness of the BPP is also limited by a high false-positive rate (50 per cent) and further research is required to elucidate whether use of the BPP with other tests (for example, Doppler studies) may improve our ability to identify compromised babies.30
New Zealand MFM Network guideline
The New Zealand MFM Network guideline for the management of suspected SGA singleton pregnancies and infants after 34 weeks gestation has recently (October 2014) been revised. This useful resource is available online.31 It provides a summary of current evidence to guide best practice with algorithms and Doppler reference charts to guide individual patient care and provide frameworks for development of local guidelines.
Other
Cervical length
In women with symptoms of preterm labour, transvaginal measurement of cervical length and has a similar ability to predict delivery within 48 hours and seven days as fetal fibronectin.32 In an analysis of 24 trials involving more than 5000 women, using a cut-off of ≤15mm appeared to be most accurate in predicting preterm birth within seven days (sensitivity 0.74 and specificity 0.89).33
Disorders of placentation
Placental location is defined at the time of the anomaly scan at 18–20 weeks. Where the placenta is seen to be low lying, placental position is reviewed in the third trimester to allow planning of
safe delivery. In women with risk factors for a morbidly adherent placenta, the index of suspicion for placenta accreta should be high and specialist opinion sought. Ultrasound findings suggesting of accreta include loss of the normal retroplacental hypoechoic zone, presence of multiple intraplacental lacunae, loss or disruption of the serosa–bladder wall interface or a focal elevation/mass of the same echogenicity as the placenta visualised beyond the uterine serosa (indicating percreta).34 35 36
Guidance procedures
Real-time ultrasound imaging improves the safety and success of procedures such as amniocentesis and chorionic villus sampling37 and has opened the door to more invasive techniques, such as percutaneous fetal blood sampling, fetal transfusion38 and other fetal interventions.
Three-dimensional imaging
Three-dimensional ultrasound imaging has been available since the 1980s, however owing to poor image quality and slow processing speeds the technique was slow to be adopted.2 Three-dimensional imaging (and real time 4D imaging) may be beneficial for assessing surface anomalies such as facial clefts and spinal defects.39 With the provision of strikingly life-like images, there is increasing demand from families to access these scans. Concern regarding possible risks of these procedures when they are being performed without medical benefit has led to the international recommendation: ‘that diagnostic ultrasound should be performed only when a medical indication exists and not solely for visualising the fetus or obtaining pictures/video clips of the fetus’.40
- Ultrasound is the most commonly used imaging modality in pregnancy.
- Assessment of fetal growth with the aim of identifying and monitoring the SGA or IUGR baby is arguably the most important role of ultrasound scanning in the third trimester.
- Babies identified as SGA and/or IUGR should be concurrently investigated with UmA, MCA and UtA Doppler in order to further stratify risk.
- Before performing or requesting imaging, consider how results will impact on management. Avoid unnecessary investigations in low-risk pregnancies.
Non-indicated use
In the low-risk or unselected population, there are no proven benefits of late pregnancy ultrasound in terms of reduction in intervention or improved perinatal outcome.41 Similarly, Doppler ultrasound of any vessel should not be used as a screening tool in unselected populations.42
Other imaging techniques
X-ray
X-ray of the fetus was reported as early as the 1920s. It has been used in attempts to determine viability, pregnancy dating, fetal and placental location, fetal bone anomalies, risk of obstructed labour and even to guide needle placement for in utero fetal blood transfusion.43 It has now been superseded by technologies obtaining real-time higher quality images without the use of ionising radiation.
Computed tomography
Owing to concerns regarding ionising radiation exposure to the fetus, computed tomography (CT) is rarely used in obstetrics. However, its use in pregnancy may be indicated for emergency non-obstetric indications, such as following high-energy trauma, pulmonary embolism and abdominal pain.
Magnetic resonance imaging
Magnetic resonance imaging (MRI) uses non-ionising radiation and is deemed safe for mother and fetus. It allows evaluation of complex fetal anatomy where structures may be better visualised than with ultrasound.44 45 It is particularly useful in assessment of the central nervous system, thorax and head and neck (allowing antenatal fetal airway assessment). MRI is also a useful adjunct in planning delivery in cases of abnormal placentation, particularly where a posterior placenta increta or percreta is suspected.46
Conclusion
Ultrasound is the most important imaging tool for evaluation of the fetus in the third trimester. Skill lies not only in performing these tests, but also the selection of women and babies who will benefit from them, the interpretation of scan data in context of the pregnancy and subsequent management. With ongoing use, hopefully, we will be able to use these ‘windows’ more effectively, as research tools to understand normal and complicated pregnancies, and clinically to improve the management and outcome of all pregnancies.
References
- Guideline for the management of suspected small for gestational age singleton pregnancies after 34 weeks gestation. www.healthpoint.co.nz/public/new-zealand-maternal-fetal-medicine-network/?solo=otherList&index=1 updated 2014.
- Benson CB, Doubilet PM. The history of imaging in obstetrics. Radiology. 2014;273(2 Suppl):S92-S110.
- Saltvedt S, Almström H, Kublickas M, Valentin L, Grunewald C. Detection of malformations in chromosomally normal fetuses by routine ultrasound at 12 or 18 weeks of gestation: a randomised controlled trial in 39 572 pregnancies. BJOG. 2006;113(6):664-674.
- Gardosi J, Madurasinghe V, Williams M, Malik A, Francis A. Maternal and fetal risk factors for stillbirth: Population based study. BMJ. 2013;346:f108.
- Bricker L, Neilson JP, Dowswell T. Routine ultrasound in late pregnancy (after 24 weeks’ gestation). Cochrane Database Syst Rev. 2008;(4):CD001451. doi(4):CD001451.
- Guideline for the management of suspected small for gestational age singleton pregnancies after 34 weeks gestation. www.healthpoint.co.nz/public/new-zealand-maternal-fetal-medicine-network/?solo=otherList&index=1 updated 2014.
- Guideline for the management of suspected small for gestational age singleton pregnancies after 34 weeks gestation. www.healthpoint.co.nz/public/new-zealand-maternal-fetal-medicine-network/?solo=otherList&index=1 updated 2014.
- Gardosi J, Francis A. Controlled trial of fundal height measurement plotted on customised antenatal growth charts. BJOG. 1999;106(4):309-317.
- Scioscia M, Vimercati A, Ceci O, Vicino M, Selvaggi LE. Estimation of birth weight by two-dimensional ultrasonography: A critical appraisal of its accuracy. Obstet Gynecol. 2008;111(1):57-65.
- Statement on normal ultrasonic fetal measurements. www.asum.com. au/newsite/files/documents/policies/PS/D7_policy.pdf updated 2001.
- Guideline for the management of suspected small for gestational age singleton pregnancies after 34 weeks gestation. www.healthpoint.co.nz/public/new-zealand-maternal-fetal-medicine-network/?solo=otherList&index=1 updated 2014.
- Gestation network growth charts. www.gestation.net/growthcharts.htm updated 2014.
- Small for gestational age fetus, investigation and management (green-top guideline no. 31). www.rcog.org.uk/en/guidelines-research-services/guidelines/gtg31/ updated 2013.
- Gardosi J, Mongelli M, Wilcox M, Chang A. An adjustable fetal weight standard. Ultrasound Obstet Gynecol. 1995;6(3):168-174.
- Weeks JW, P. Fetal macrosomia: Does antenatal prediction affect delivery route and birth outcome? Obstet Gynecol. 1995;173(4):1215-1219.
- Karsdorp VHM. Clinical significance of absent or reversed end diastolic velocity waveforms in umbilical artery. Lancet. 1994;344(8938):1664-1668.
- Guideline for the management of suspected small for gestational age singleton pregnancies after 34 weeks gestation. www.healthpoint.co.nz/public/new-zealand-maternal-fetal-medicine-network/?solo=otherList&index=1 updated 2014.
- Alfirevic Z, Stampalija T, Gyte GM. Fetal and umbilical doppler ultrasound in high-risk pregnancies. Cochrane Database Syst Rev. 2013;11:CD007529.
- Guideline for the management of suspected small for gestational age singleton pregnancies after 34 weeks gestation. www.healthpoint.co.nz/public/new-zealand-maternal-fetal-medicine-network/?solo=otherList&index=1 updated 2014.
- Oros D, F. Longitudinal changes in uterine, umbilical and fetal cerebral doppler indices in late- onset small-for- gestational age fetuses. Ultrasound in Obstetrics & Gynecology. 2011;37(2):191.
- Hernandez-andrade E. Cerebral blood flow studies in the diagnosis and management of intrauterine growth restriction. Curr Op Obstet Gynecol. 2013;25(2):138-144.
- Guideline for the management of suspected small for gestational age singleton pregnancies after 34 weeks gestation. www.healthpoint.co.nz/public/new-zealand-maternal-fetal-medicine-network/?solo=otherList&index=1 updated 2014.
- Ghidini A, Locatelli A. Monitoring of fetal well-being: Role of uterine artery doppler. Semin Perinatol. 2008;32(4):258-262.
- Oros D, F. Longitudinal changes in uterine, umbilical and fetal cerebral doppler indices in late- onset small-for- gestational age fetuses. Ultrasound in Obstetrics & Gynecology. 2011;37(2):191.
- Oros D, F. Longitudinal changes in uterine, umbilical and fetal cerebral doppler indices in late- onset small-for- gestational age fetuses. Ultrasound in Obstetrics & Gynecology. 2011;37(2):191.
- Mari G, Deter RL, Carpenter RL, et al. Noninvasive diagnosis by doppler ultrasonography of fetal anemia due to maternal red- cell alloimmunization. N Engl J Med. 2000;342(1):9-14.
- Magann EF, Sandlin AT, Ounpraseuth ST. Amniotic fluid and the clinical relevance of the sonographically estimated amniotic fluid volume: Oligohydramnios. Journal of Ultrasound in Medicine. 2011;30(11):1573-1585.
- Lalor JG, Fawole B, Alfirevic Z, Devane D. Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database Syst Rev. 2008;(1):CD000038. doi(1):CD000038.
- Figueras F, Gardosi J. Intrauterine growth restriction: New concepts in antenatal surveillance, diagnosis, and management. Obstet Gynecol. 2011;204(4):288-300.
- Guideline for the management of suspected small for gestational age singleton pregnancies after 34 weeks gestation. www.healthpoint.co.nz/public/new-zealand-maternal-fetal-medicine-network/?solo=otherList&index=1 updated 2014.
- Boots AB, Sanchez-Ramos L, Bowers DM, Kaunitz AM, Zamora J, Schlattmann P. The short-term prediction of preterm birth: A systematic review and diagnostic metaanalysis. Am J Obstet Gynecol. 2014;210(1):54.e1-54.e10.
- Boots AB, Sanchez-Ramos L, Bowers DM, Kaunitz AM, Zamora J, Schlattmann P. The short-term prediction of preterm birth: A systematic review and diagnostic metaanalysis. Am J Obstet Gynecol. 2014;210(1):54.e1-54.e10.
- Riteau A-S, Tassin M, Chambon G, et al. Accuracy of ultrasonography and magnetic resonance imaging in the diagnosis of placenta accreta. PLoS ONE. 2014;9(4).
- Belfort MA. Placenta accreta. Obstet Gynecol. 2010;203(5):430-439.
- Comstock C, Bronsteen R. The antenatal diagnosis of placenta accreta.BJOG. 2014;121(2):171-182.
- Crandon AJ, Peel KR. Amniocentesis with and without ultrasound guidance. BJOG. 1979;86(1):1-3.
- Berry SM, Stone J, Norton ME, Johnson D, Berghella V. Fetal blood sampling. Obstet Gynecol. 2013;209(3):170-180.
- Merz E, Abramowicz JS. 3D/4D ultrasound in prenatal diagnosis: Is it time for routine use? Clin Obstet Gynecol. 2012;55(1):336-351.
- Abramowicz JS, B. The safe use of non-medical ultrasound. Ultrasound in Obstetrics and Gynecology. 2009;33(5):617-620.
- Bricker L, Neilson JP, Dowswell T. Routine ultrasound in late pregnancy (after 24 weeks’ gestation). Cochrane Database Syst Rev. 2008;(4):CD001451. doi(4):CD001451.
- Goffinet F, Paris-llado J, Nisand I, Bréart G. Umbilical artery doppler velocimetry in unselected and low risk pregnancies: A review of randomised controlled trials. BJOG. 1997;104(4):425-430.
- Benson CB, Doubilet PM. The history of imaging in obstetrics. Radiology. 2014;273(2 Suppl):S92-S110.
- Benson CB, Doubilet PM. The history of imaging in obstetrics. Radiology. 2014;273(2 Suppl):S92-S110.
- Patenaude Y, Pugash D, Lim K, et al. The use of magnetic resonance imaging in the obstetric patient. JOGC. 2014;36(4):349.
- Patenaude Y, Pugash D, Lim K, et al. The use of magnetic resonance imaging in the obstetric patient. JOGC. 2014;36(4):349.







Leave a Reply