Female fertility is extremely fragile, especially with respect to ovarian function and egg quality, and should never be taken for granted. The detrimental effects of female age on fertility and egg quality are predictable, relentless and unfortunately still currently resistant to medical intervention. Sadly, for many women towards the end of the reproductive lifespan, the opportunity to have a family, with their own genetically related children, has not been possible until now. Likewise, young women with cancer or other serious diseases, who require chemotherapy, radiotherapy or surgery, are at extremely high risk of infertility or even sterility as a consequence of this life-saving treatment.1 2
Over the last 20–30 years there has been an increasing commitment from the scientific and medical community to develop strategies to protect and preserve fertility, and thus expand reproductive opportunity in the future for women whose fertility is seriously compromised by increasing age, social circumstance or medical treatment.
The scientific advances in vitrification and survival of oocytes (eggs) and the increasing accessibility of egg freezing programs are now able to provide new hope for these women; but is it realistic hope or is it false hope? Just because we can now offer elective (non-medical) egg freezing, should we?
The medical, technical, ethical, social and economic implications provide rich fodder for discourse. Healthy debate should avoid personal prejudice and social judgement, and focus instead on the bigger picture. This includes both recognising the basic liberty right to have the opportunity for a family and also understanding the importance of reproductive autonomy.
Female ageing and fertility
A reduction in egg number and the increasing genetic and metabolic fragility of eggs are both implicated in normal senescence. However, the importance of the biological clock has become more relevant in contemporary society, as we partner later and have our children later, with the associated age-related risks of reduced fertility, miscarriage and other complications. There is a common misconception (pardon the pun), that fertility treatment and IVF will help age- related infertility, but to date no scientific treatment available in a clinical setting has been shown to improve egg quality.
The science of egg freezing
Until fairly recently, the technique employed for freezing and thawing eggs (namely slow-freezing) resulted in suboptimal survival and hence numerically little opportunity for conception. Although the first birth from a frozen-thawed egg was reported in 19863, further success has been elusive until the last 10–15 years. The high water content in eggs provides a challenge and slow freezing is associated with development of ice crystals, chilling injury and osmotic damage contributing to poor viability after thawing.4 However, the development of vitrification, which uses high freezing and cooling rates to avoid ice crystal formation, means that survival of eggs exceeds 80–90 per cent.6 Vitrification is now almost universally used in egg freezing programs worldwide.
Motivations for egg freezing
While there is a populist view that the average woman who is interested in egg freezing is career-driven, with no time to conceive at a reproductively appropriate time, the reality is very different: for many women, the motivation stems from a current lack of opportunity to conceive, despite a strong desire to have a family or at least the choice to have a family. Most women are not in a relationship, not in a suitable relationship or are recently separated, having expected to have children with their ex-partners.5 6
The success of egg freezing
With excellent survival now established as a result of vitrification, it is reassuring to know that, once an egg survives the freezing-thawing process, it behaves as a fresh egg, with comparable fertilisation, implantation and pregnancy rates7, and no apparent increase in abnormality rate.8 Frozen eggs are used in most of the big egg donation programs in Europe and the US with great success.
However, it is extremely important to understand that, exactly as with fresh eggs, there is a high rate of expected attrition with the progression from mature egg to usable embryo. Approximately 50–60 per cent of eggs will fertilise and then there is further attrition during development of the early embryo. So for every ten eggs that are frozen and thawed, two to four will develop to early embryo stage (day 2–3) and possibly only one to two embryos will progress to the blastocyst stage (day 5).
It is conservatively estimated that between 15 and 25 eggs would be required for one baby in a woman who undergoes freezing under the age of 38 years9, and this may involve one or more cycles of stimulation and cryopreservation.
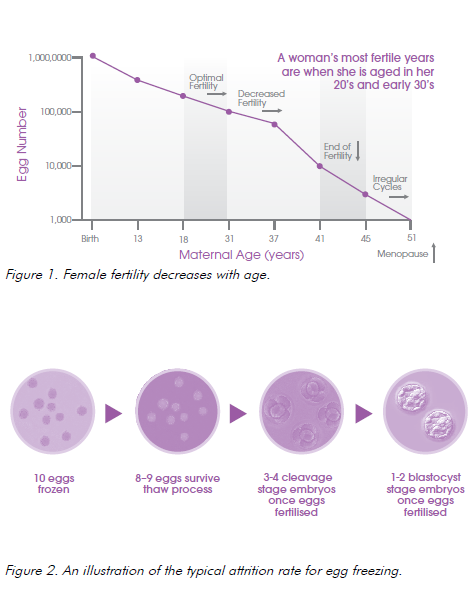
While the average number of oocytes obtained per cycle is 10–15, consideration of age, anti-Mullerian hormone (AMH) and follicle stimulating hormone (FSH) level and ultrasound assessment of follicles, can all assist in predicting the result for an individual woman.
As women age, they will respond to ovarian stimulation with fewer egg numbers, so on the whole, egg freezing is not offered to women over 38 years of age. However, there is room to allow individualisation of decision-making, such that if a woman older than 38 has excellent ovarian function with a high yield of eggs expected, the technique can be considered.
The biggest challenge when counselling women who request discussion about egg freezing is to ensure that they understand that even with a good number of eggs frozen, at most this technology will offer a small, finite number of additional opportunities to conceive in the future. So this must not be relied on as a form of reproductive insurance and women need to understand the perils of making subsequent life decisions to delay fertility on the basis of having some frozen eggs.
Notwithstanding the words of caution, egg freezing is acknowledged as a technique that has come of age and is no longer viewed as experimental.10 This may be why some US tech companies now offer the opportunity for egg freezing to their female employees, prompting vigorous debate about the competing motivations of coercion versus enlightened employment packages.
Box 1. AMH
- produced by small follicles in ovary;
- used as surrogate marker of primordial follicles and hence ovarian reserve;
- much variation between assays;
- does NOT reflect quality of oocytes;
- Can still conceive successfully with very low level; and gives very vague indication of expected yield in ovarian stimulation
Timing for egg freezing
Paradoxically, in order to maximise egg numbers and offer a substantial opportunity to later conceive, egg freezing is best undertaken at a relatively young age, when fertility or even future fertility is not yet a consideration. A woman of 25–30 years of age will generally produce a much larger number of eggs, with more robust genetics and thus far greater implantation potential, than a woman over the age of 35.
Most women, however, who come to have discussion are already in their mid-late 30s or even over 40. They come because life has not turned out the way they would wish, they are not in a position to have a baby, either as a single woman or with a partner, and often they are already grieving their loss of fertility potential. In this situation, we need to consider the benefits of going ahead versus not going ahead. While the chance may be relatively low of obtaining enough good quality eggs, it certainly provides more opportunity than NOT going ahead. Also, many women feel reassured by having done everything they can to aid future fertility and this feeling of control is very important to them, as it may help reduce the desperation to find someone to reproduce with.
Ideally, however, we should consider a societal shift. If we could turn things around and consider the option of egg freezing at a much younger age, viewed as an opportunity to plan ahead, just in case things don’t work out, there would be much more positivity associated with the concept, not to mention a more realistic chance of success.
The practicalities of egg freezing
Assessment
Women who are interested in maximising future fertility need to be evaluated for any reproductive risk factors, significant past history and relevant family reproductive history. It is possible to assess current ovarian function and get some idea of ovarian reserve by a combination of endocrine parameters (day 2 FSH and AMH) and early follicular phase ultrasound. Interpretation of AMH levels is extremely problematic and caution should be employed (see Box 1). Many women are reported as having impaired ovarian reserve, but this only reflects the normal attrition associated with ovarian ageing.
The evaluation should include discussion of the woman’s fertility aspirations, plans for the future and other options, including consideration of getting on and having a baby (with donor sperm if required) in the late 30s or early 40s. Counselling should be performed, including a frank discussion of expected yield and how much genuine additional opportunity is likely to be provided by uptake of the process. Access to a reproductive unit counsellor as well as a medical specialist may be useful.
The process
Technically, an egg freezing cycle is much like an IVF cycle, involving hormone stimulation with FSH for 10–14 days to stimulate development of multiple follicles to maturity. An additional injection of
a gonadotrophin-releasing-hormone antagonist (GnRH) is used for the last 3–6 days of stimulation to prevent premature ovulation of the more mature follicles. An injection of recombinant human chorionic gonadotrophin (hCG) is given 37 hours before the procedure to trigger completion of meiosis I and initiate meiosis II, and to prepare the follicles for imminent release. The oocyte retrieval takes place about day 12–16 of the cycle, usually under sedation, via a trans-vaginal approach. Patients are discharged home approximately an hour after the procedure, with instructions to take it gently for a day or so.
The risks
Fortunately, undergoing ovarian stimulation and oocyte retrieval does not use up eggs or predispose to earlier menopause. The hormone stimulation is associated with an extremely small risk of thrombosis (of the order of 1/5000) or infection (1/5000). Ovarian hyperstimulation syndrome has fortunately been reduced to an almost negligible risk, thanks to careful practice and the availability of an alternative (GnRH agonist) trigger injection if required. The common symptoms of bloating and fullness resolve over a few days and patients expect to feel completely back to normal within 7–14 days after the procedure. As discussed, the major risk associated with this is the misguided reliance on a small supply of frozen eggs as a guarantee of future fertility.
The cost
There is no Medicare reimbursement for non-medical egg freezing and the costs vary between centres, but are in the vicinity of $8000–12 000 for a cycle, with storage costing $200–500 per year.
Conclusions
The best chance of having a baby involves spontaneous fertility in the mid to late 20s or very early 30s. This opportunity may not be available to some women for a variety of reasons and thus fertility preservation by egg freezing may offer these women a realistic chance of having their own child in the future. The magnitude of the additional opportunity will be related to the age of the woman at the time of the freezing, the number and the quality of the eggs frozen expected benefit, but we must make sure that we do not judge them. For some women, any extra chance of future fertility is better than none, as long as there is no false hope which affects subsequent decision- making. The value of allowing women the opportunity to take some (even just a tiny bit) of control over their reproductive future cannot be underestimated.
It is likely that within the next few years, we will see more young women adopting a proactive approach, considering egg freezing before the commencement of the fertility decline. It is imperative that this does not perpetuate the societal pressures that predispose to high maternal age. We must continue to strive for better conditions for combining early childbearing with other professional aspirations and life goals.
References
- Stoop D, Nekkebroeck J, Devroey P. A survey on the intentions and attitudes towards oocyte cryopreservation for non-medical reasons among women of reproductive age. Hum Reprod 2011b;26: 655-661.
- Gold E, Copperman K, Witkin G, Jones C, Copperman AB. P-187: a motivational assessment of women undergoing elective egg freezing for fertility preservation. Fertil Steril 2006;86:S201.
- Chen C. Pregnancy after human oocyte cryopreservation. Lancet 1986;1:884-6.
- Woods EJ, Benson JD, Agca Y, Critser JK. Fundamental cryobiology of reproductive cells and tissues. Cryobiology 2004;48:146-56.
- Stoop D, Nekkebroeck J, Devroey P. A survey on the intentions and attitudes towards oocyte cryopreservation for non-medical reasons among women of reproductive age. Hum Reprod 2011b;26: 655-661.
- Gold E, Copperman K, Witkin G, Jones C, Copperman AB. P-187: a motivational assessment of women undergoing elective egg freezing for fertility preservation. Fertil Steril 2006;86:S201.
- Rienzi L, Cobo A, Paffoni A, Scarduelli C, Capalbo A, Vajta G, et al. Consistent and predictable delivery rates after oocyte vitrification: an observational longitudinal cohort multicentric study. Hum Reprod 2012;17:1606-12.
- Noyes N, Porcu E, Borini A. Over 900 oocyte cryopreservation babies born with no apparent increase in congenital anomalies. Reprod Biomed Online 2009;18:769-76.
- Practice Committees of the American Society for Reproductive Medicine and the Society for Assisted Reproductive Technology. Mature oocyte cryopreservation: a Fertil Steril 2013;99:37-43.
- The Practice Committee of the Society for Assisted Reproductive Technology (SART) and the Practice Committee of the American Society for Reproductive Medicine (ASRM). Essential elements of informed consent for elective oocyte cryopreservation: a Practice Committee opinion. Fertil Steril 2008;90:S134.






Leave a Reply