The concept of transfusing blood from one human to another was first explored almost two hundred years ago, and the very first clinical setting was in treatment of postpartum haemorrhage.1 The trials were carried out by James Blundell, an English obstetrician desperate to help the many women who succumbed to massive bleeding in an era when there was little else to offer. Blundell’s transfusions were carried out before the ABO blood typing system was recognised, yet five women survived following his radical treatment. Later in the nineteenth century, a letter in the Lancet written by Mr William Highmore detailed the case of a woman who died from a postpartum haemorrhage. Highmore speculated that had the woman’s blood been collected and transfused, it might have saved her life. The concept of auto-transfusion was thus envisioned by those providing care during childbirth.
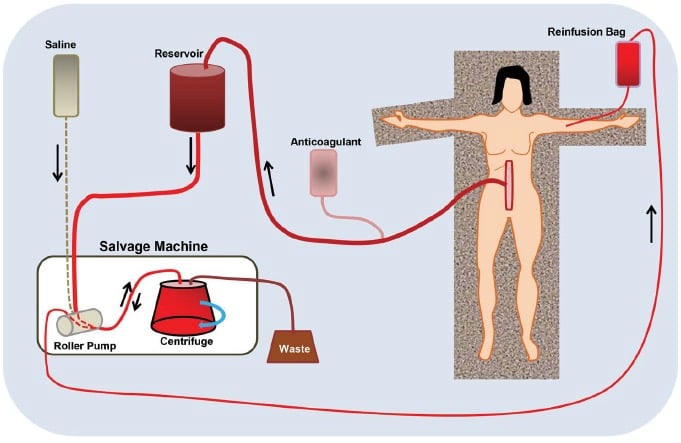
Figure 1. Schematic of a typical cell salvage system.
Blood in the field of surgery
Cell salvage – the process by which blood lost during surgery is harvested for autologous transfusion – has evolved since its introduction in the 1960s. It is now recognised that blood lost during surgery has high concentrations of inflammatory mediators, free haemoglobin and cellular debris. To deal with these potential contaminants, washing and preparation of the saved blood is necessary. The four key elements of cell salvage and subsequent transfusion are:
- collection of the blood;
- addition of an anticoagulant;
- processing to concentrate and harvest the blood cells; and
- autologous transfusion.
The typical layout of a cell salvage system is shown in Figure 1.
The first step in cell salvage, collection of the blood, might seem simple, but there is considerable potential for harm in the process. Suction pressure applies mechanical forces to blood cells and shearinduced haemolysis occurs with high suction pressures. The risk of this can be increased markedly by the shape of the suction tip, as well as co-aspiration of air that causes bubble formation and cell damage. For these reasons, suction catheters specifically designed for salvage should be used, with lower negative pressures than usual. To minimise mixing with air, the catheter tip should remain below the surface of the blood pool while aspirating. Blood should also be suctioned as quickly as possible to avoid prolonged contact with the wound surfaces, thereby minimising exposure to factors initiating the coagulation cascade.
The aspirated blood is mixed with an anticoagulant to prevent clot formation in the suction tubing, reservoir and processing drum. Clotting in the system reduces the yield of cells and can necessitate changing of the tubing if it clogs. The anticoagulant typically used is heparin, because of its low cost and ready availability, but citrate is also appropriate. Heparin concentrations of 30 000 units per 1000ml of saline are ideal for the purpose, and this can be mixed with the blood aspirate in a ratio of about 15ml for every 100ml of aspirated blood. There is little downside to adding too much heparin, since the large heparin molecule is easily removed from the tranfusate during the processing stage.
Before the mixture of salvaged blood and anticoagulant moves to the processing bowl, it is stored in a collection reservoir. Because of the dynamics of processing, the volume of the reservoir needs to be three to four times greater than the volume of the processing bowl. The collection reservoirs are lined with a filter where the pore size is between 50 and 100μm. The pore size makes avoidance of clotting very important, hence the desirability of the anticoagulant mixture.
The heart of the cell salvage unit is a centrifuge drum. The blood mixture is pumped from the reservoir and enters the processor through a central ‘straw’ (see Figure 2). The blood flows to the floor of the processor as the unit spins, moving under centrifugal force. Since the red cells have greater mass than other components of the mixture, they separate and spread out around the walls of the processor bowl. The separation of red cells from other plasma components (including heparin molecules) depends upon a balance of hydrostatic forces from the blood being pumped in and the centrifugal force generated by rotation of the bowl. When the balance is correct, the non-cellular (plasma) component of the mixture exits the bowl into the waste receptacle and the red cells remain in the bowl, allowing them to be isolated. Once the initial processing is complete, the bowl contents are washed with saline until the exiting waste appears clear to visual inspection. When the washing is complete, the roller pump is reversed, allowing collection of the washed and packed red cells into a reinfusion bag. Resuspension of the washed cells in saline yields a reinfusion solution with a haematocrit of between 50 and 80 per cent. The use of a micro-aggregate blood filter at transfusion, with a pore size of 40μm, can trap and remove bacteria and amniotic fluid components.
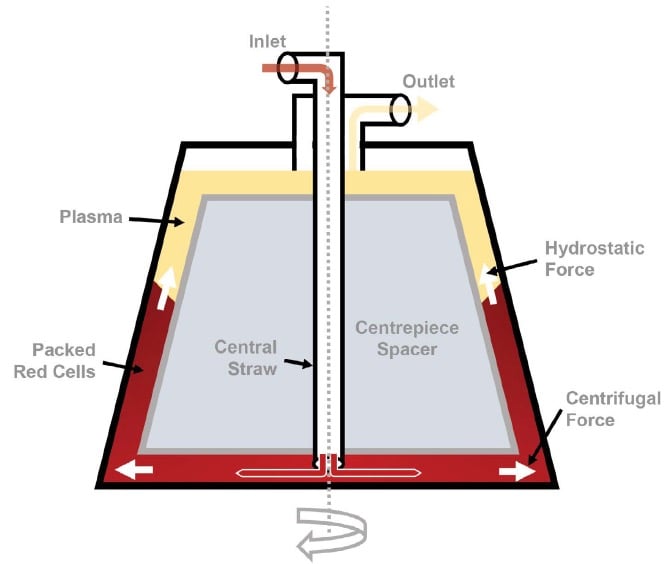
Figure 2. A cross-section of the cell salvage centrifuge drum, showing the separation process.
Cell salvage in a nutshell
- If significant blood loss is anticipated, consult the patient and liaise with the anaesthetic team about the possibility of cell salvage
- Use a sucker and tubing specifically designed for the cell salvage
- Avoid aspirating from the surface of the blood pool as mixing the aspirate with air can result in cell damage
- Avoid aspirating amniotic fluid (in obstetric cases) to minimise the risk of amniotic fluid embolism
- Salvaged blood does not contain platelets or coagulation factors. These need to be replaced as indicated
- The use of cell salvage in oncology remains uncertain.
Complications of cell salvage
There are a number of potential complications with the use of cell salvage. These include microembolism (caused by micro-aggregates); fat and air embolism; disturbances of electrolytes; fever; haemolysis; and renal injury from free haemoglobin. A particular complication known as ‘salvaged blood syndrome’ can occur when the salvaged cells are diluted in large volumes of saline. This leads to deposition of cellular aggregates in the fixed bowls of the processor, and has been associated with triggering of the intravascular coagulation sequence, increasing permeability of the microvascular beds leading to lung injury and even renal failure.
It is also important to understand that salvaged blood does not contain any platelets or coagulation factors. This is a critical consideration in the management of massive haemorrhage and means that infusion of cryoprecipitate, fresh-frozen plasma and platelets will be required for additional support in these circumstances.
Figure 3. One of the authors, right, with the cell
saver equipment.
Cell salvage in obstetrics
Although the concepts of blood transfusion and cell salvage were initially inspired by massive obstetric haemorrhage, concerns about amniotic fluid embolism and retransfusion of fetal debris led to great caution in its use in pregnant women. However, the contribution of bleeding to maternal death and debility has promoted re-evaluation of the role of cell salvage in these settings.
Because of the rarity of amniotic fluid embolism, study of its incidence in a setting of cell salvage is almost impossible. When washed blood that had been filtered using leucocyte-depletion filters was studied, it revealed levels of fetal squames similar to those normally found in maternal blood in the postnatal period.2 This provides reassurance, but is obviously only indirect evidence. However, to date, there are no reports of amniotic fluid embolism associated with the use of salvaged blood potentially contaminated with amniotic fluid.
Liumbruno’s group have reviewed the literature and reported that cell salvage appears to be as safe as donor blood transfusion in the management of placenta accreta.3 4 They concluded that complications associated with cell salvage were unrelated to the salvage itself. These included endometritis, staphylococcal pneumonia and hypotension – all could be explained by other factors associated with the surgery. Irrespective, caution should be exercised including minimising aspiration of amniotic fluid, and use of leucocyte depletion filters during transfusion. If leucocyte depletion filters are used, it is important not to use pressure bags or other pressurisation devices during the transfusion as this can cause the release of vasoactive substances that can lead to hypotensive reactions.
Cell salvage in gynaecology The use of cell salvage in gynaecological settings should follow protocols associated with surgery in non-pregnant women. A typical setting might be hysterectomy for a massive fibroid uterus, where heavy blood loss is anticipated. It is important to remember that associated bowel injury presents a risk of infection and cell salvage should be commenced after decontamination and with the use of antibiotic cover. Conditions such as sickle cell disease and thalassaemia are also relative contraindications and might require special considerations.
The use of cell salvage in the setting of surgery for known or suspected malignancy is more problematic. Manufacturers of cell salvage systems typically warn against use because of the risk of reinfusion of malignant cells and the potential for assisted metastasis. That said, studies of cell salvage in radical surgery for prostatic cancer have not been reported to show any increase in the risk of recurrence or any change in survival statistics. National Institute for Health and Care Excellence guidelines in the UK support the use of cell salvage in surgery for urological malignancy, but suggest the use of leucocyte depletion filters and consideration of irradiation of the salvaged blood before reinfusion. To date, there are few data to guide usage in surgery for gynaecological cancers, so the issue remains unresolved.5
Conclusion
The ideas of blood transfusion and cell salvage came from doctors struggling to treat women who had massive postpartum haemorrhage. The increasing incidence of placenta accreta, with its risk for difficult-to-control bleeding at delivery, has led to a re-examination of cell salvage techniques in obstetrics. The available evidence is that cell salvage, when employed judiciously and with care, has a role to play. The use of cell salvage in gynaecological surgery where heavy blood loss is anticipated is also supported. The area where caution should be employed is in surgery for gynaecological malignancy.
References
- Kuppurao L, Wee M. Perioperative cell salvage. Contin Ed Anaes Crit Care Pain 2010; 10 (4):104-8.
- Waters JH. Intraoperative blood recovery. ASAIO Journal 2013; 59:11-17.
- Liumbruno GM, Meschini A, Liumbruno C, Rafanelli D. The introduction of intra-operative cell salvage in obstetric clinical practice: a review of the available evidence. Eur J Obstet Gynecol Reprod Biol 2011; 159:19-25.
- Liumbruno GM, Liumbruno C, Rafanelli D. Intraoperative cell salvage in obstetrics: is it a real therapeutic option? Transfusion 2011; 51:2244-56.
- Nagarsheth NP, Sharma T, Shander A, Awan A. Blood salvage use in gynecologic oncology. Transfusion 2009; 49 (10):2048-53.







hello
I am Anaesthetic Tech student really very good information for understand of concept of cell salvage, really interesting ,very helpful for my career .Thank you