Management of bleeding is a fundamental consideration in all surgery, so much so that many surgeons take it for granted. Effective control of bleeding is necessary to minimise the adverse physiological sequelae of surgery that affect recovery, including the need for transfusion, and also to optimise the view of the surgical field thereby reducing the risk of surgical complications. Being able to see what you are operating on reduces time taken for surgery, minimises the risk of inadvertent injury to the patient and reduces the chance of needlestick injuries. Key strategies for adequate haemostasis include ensuring the best pre-operative preparation for patients, good anaesthetic support and attention to surgical technique. Yet, despite every effort, bleeding can still complicate surgical procedures. Haemostatic agents are commonly employed to assist with the control of bleeding; this article aims to provide some background to the physiological basis of local and systemically administered haemostatic agents.
Haemostasis
If the aim is to stop bleeding, it is important to understand the normal physiological mechanisms that lead to clotting. Haemostasis results from a complex interplay of factors involving the blood vessel wall, coagulation factors and platelets (see Figure 1). Primary haemostasis promptly follows injury to the vessel endothelium: the injured vessel constricts, platelet factors are released and platelets stick together, and form a soft plug that temporarily reduces further blood loss. Platelets aggregate because they are activated by thrombin.
Once activated, the platelets release a number of potent vasoactive factors that play a role in the ‘coagulation cascade’ (see Figure 2). Generation of fibrin provides a framework over the relatively loose platelet plug, stabilising it. Two different arms in the coagulation cascade – the intrinsic pathway (initiated by endothelial damage) and the extrinsic pathway (initiated by transection of vessels) – both lead to a final common pathway, shown diagrammatically in Figure 2. It is important to recognise that the cascade can stall when one of the participant molecules is absent, such as can occur in the congenital haemophilias, or with the action of warfarin, and when the concentration of platelets is low.
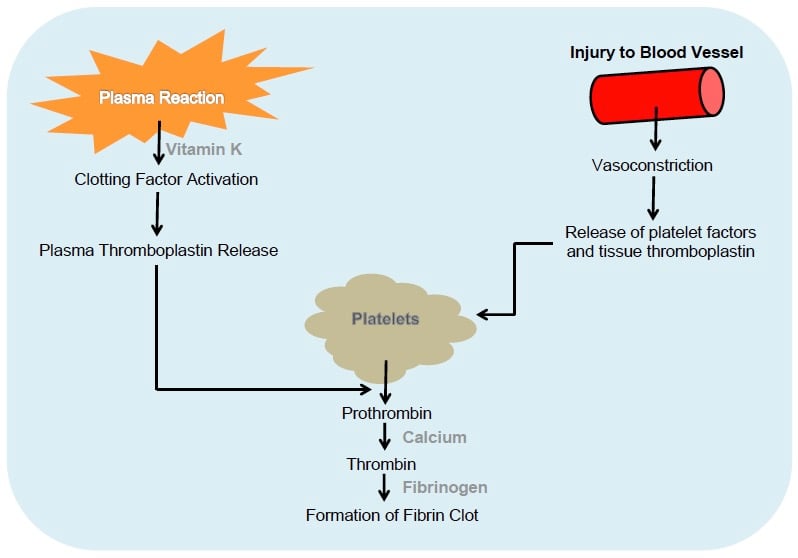
Figure 1. The physiological mechanisms involved in haemostasis.
Surgical haemostasis
There are a number of approaches to haemostasis during surgery. Primary methods include mechanical actions, such as ligation of vessels and direct pressure with packs and gauzes, and the use of energies, such as diathermy (either monoor bipolar), ultrasound and laser. However, these methods will not necessarily achieve haemostasis to a suitable level and other methods may need to be considered. These may be either topical or systemic agents.
The topical agents include:
- passive haemostats based on collagen, cellulose and gelatins;
- active agents based on thrombins; and
- sealants such as fibrin-based sealants and synthetic glues.
The systemic agents include tranexamic acid and activated factor VII.
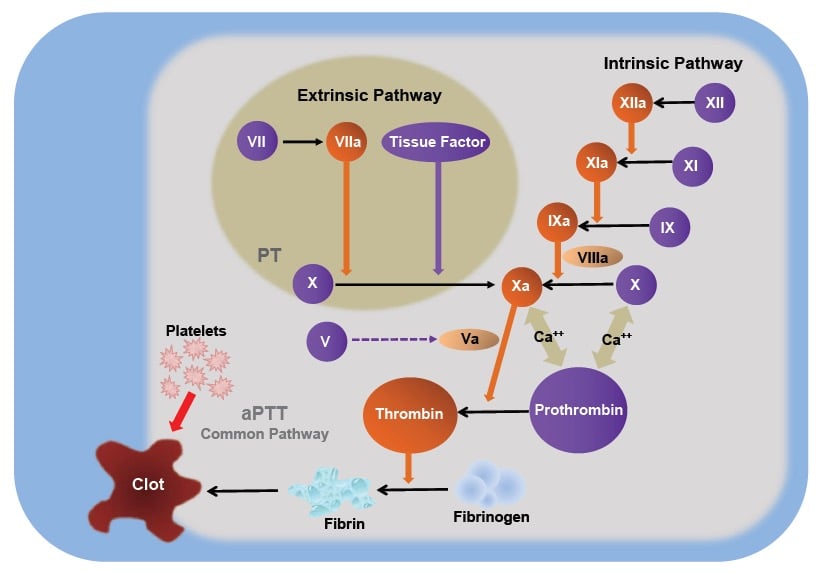
Figure 2. The coagulation cascade.
Passive topical haemostats
Passive topical haemostats act by providing a physical framework to which platelets can aggregate, allowing a clot to form over them. There are several passive haemostatic agents available, and they come in differing forms of application: ‘fleeces’ and sponges, or gauze-like sheets being the typical presentation.
Active haemostats have an intrinsic biological function that directly activates the coagulation cascade, with the intent of forming a clot at the bleeding site. These agents contain thrombin, part of the final common pathway that converts fibrinogen to fibrin, and have the advantage of bypassing the general cascade and proceeding straight to fibrin generation, an important consideration when there is disruption of the components of the coagulation cascade. Thrombin is also a component of the common sealant preparations.
By providing a framework around which platelets can adhere and aggregate, passive haemostats not only act as a physical barrier that impedes the flow of blood, but they also allow for the activation of platelets by providing a matrix for platelet adhesion and accelerating the formation of the platelet plug that forms the basis of the fibrin clot. This class of haemostats includes agents based on collagen, cellulose, gelatin and polysaccharide spheres. Haemostatic agents based on cellulose (or, more formally, oxidised regenerated cellulose [ORC]) are commonly used in operating theatres in Australia and New Zealand, and have been available for decades. Although these agents promote clot formation through contact activation, the fine detail of their exact mechanism is still not known. Commonly used ORC-based haemostats include SurgiCel™, which is available as a waxy gauze sheet, and Fibrillar™ (both from Ethicon), which comes as a cotton wool-like pad. Stable at room temperature, these agents can remain on the shelves of operating theatres. When ORCs are applied topically, they absorb blood and form a gel covering the site of the injury to the vessel. Contact with blood also leads to the breakdown of cellulose to cellulosic acid, which lowers the pH to cause localised vasoconstriction. Because their functional ability is reduced with exposure to saline, they should be used in a dry form; this has the advantage that they can be cut into shapes to fit specific spaces.
Cellulose-based products are not particularly effective in controlling bleeding from larger arteries, but are ideal for the control of bleeding from capillary beds and small veins.
Gelatin-based haemostatic agents are available as sponges or powders that also can be stored at room temperature. The first of these (GelFoam™ Pfizer) was used in 1945. Some gelatin-based sealants consist of a matrix made of gelatin with a thrombin component – when applied to a bleeding surface, the granules in the matrix allow high concentrations of thrombin to react with the patient’s fibrinogen thus forming a stable fibrin clot. The granules in the matrix swell as blood moves through the matrix further reducing blood flow and also acting as a tamponade, which conforms to the shape of the injured vessel. An example of this is FloSeal™ (Baxter), which is mixed and prepared in the operating theatre. The fluid nature of these haemostats makes them useful for cavities and irregular surfaces. However, a number of rare adverse reactions have been noted, including allergic reactions to the gelatin and fever.
Other haemostats that are not as widely used include collagen-based agents and polysaccharide microspheres.
Passive topical haemostats are useful in heavier oozing because their absorptive capacity increases mass to provide a tamponade. This absorptive tendency also means that areas of the agent that have not participated directly in haemostasis can ‘mop up’ fluid effusion. However, this effect is not always completely beneficial and expanded agents can put pressure on nearby structures such as nerves or the ureter. The presence of these agents can also lead to confusion if subsequent imaging is undertaken, where it may be misidentified as an abscess formation or even a tumour. Rarely, residual agents can promote granulation and actually slow healing. For these reasons, judicious use is always recommended.
Active haemostatic agents
Active haemostatic agents promote clot formation by participating directly in the coagulation cascade. These agents include thrombin and products that contain thrombin. Thrombin has been used for many years, with products containing thrombin derived from cattle coming on to the market in the 1970s. Thrombin is at the end of the final common pathway, so its use overcomes situations where clotting factors are absent. It also means that thrombin is more likely to be effective in the setting of a coagulopathy, or when patients have been using anti-platelet or other anticoagulants. Thrombin can be used in conjunction with other topical agents and this combination may increase the efficacy of haemostasis, as mentioned above.
Sealant agents
As their name suggests, sealants form a physical barrier that occludes flow from injured vessels. Several different types of sealants have been developed: fibrin-based sealants, cyanoacrylates, PEG polymers and albumen with glutaraldehyde.
Fibrin sealants, such as Tisseel™ (Baxter), are designed to mimic the conversion of fibrinogen to fibrin thus forming a stable clot which assists haemostasis. They contain fibrinogen and thrombin, and some may also contain antifibrinolytic agents, calcium chloride and Factor XIII. Tisseel is a pooled human plasma compound that requires frozen storage and warming for use. It is designed for use when trying to control diffuse ooze over a large area in a surgical field. It is not so useful when dealing with heavy bleeding, as might be encountered from an artery. However, when there is fibrin deficiency or the patient has been treated with heparin, it remains effective.
TXA and activated factor VII
Tranexamic acid (TXA) is a synthetic analogue of lysine, an amino acid that works by reducing fibrin breakdown (fibrinolysis). TXA blocks formation of the enzyme plasmin from its precursor molecule plasminogen, thus reducing breakdown of fibrin that has been generated during the coagulation cascade. Most doctors working in obstetrics and gynaecology are familiar with TXA. Systematic reviews have provided strong evidence that the use of TXA in the management of surgical bleeding is effective in reducing the need for blood transfusion. However whether it increases the risk of thromboembolic side effects remains unresolved.1
Recombinant activated factor VII (fFVIIa) should be reserved for patients with massive haemorrhage due to its cost, as studies have shown it to be cost effective only in patients who are likely to need a large amount of blood.2
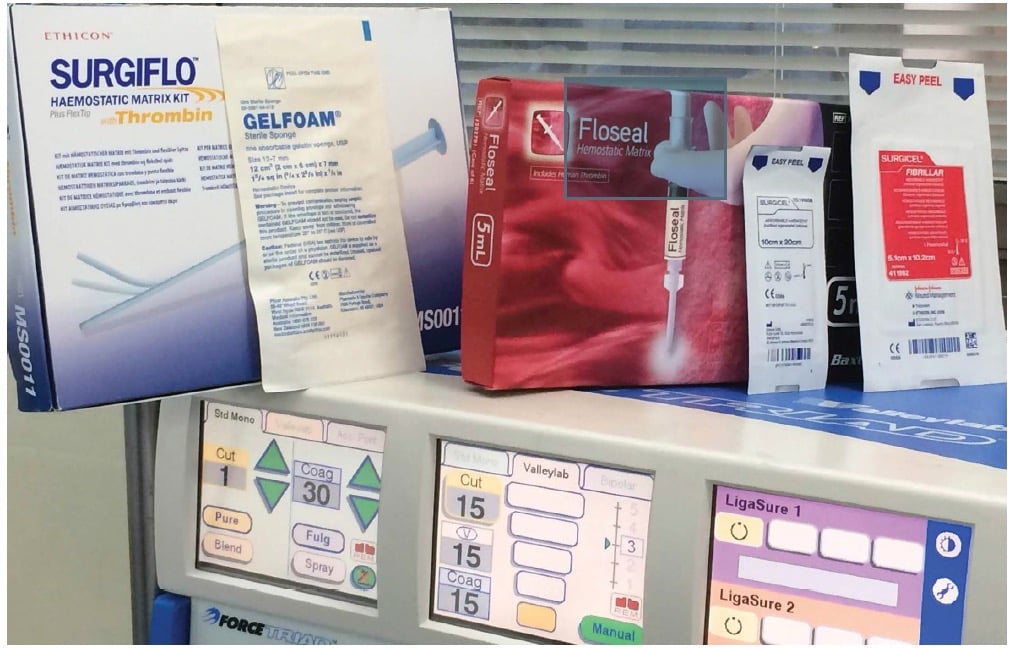
Figure 3. A selection of the haemostatic products available on the market.
How do I choose?
Dealing with surgical bleeding begins long before the operation itself, with correct patient selection – always perform the most appropriate operation for the right patient. Optimising the patient’s condition before surgery is critical and this includes managing any medications or supplements that might adversely affect haemostasis. Good anaesthetic management during surgery, including attention to hydration, will greatly help. The primary approach to minimising bleeding is good surgical technique and selective use of sutures, ligations, clips and cautery. However, despite all of this, bleeding can still occur and topical or systemic haemostatic agents may be required. Cellulose-based topical haemostats have the advantage that they can be manipulated through laparoscopic ports. The use of foams can cover larger areas, but are less effective with brisk bleeding. Thrombin-based haemostats are similarly useful in a laparoscopic setting and can control bleeding over a large area. If things continue to go wrong, the use of systemic agents, such as TXA and fFVIIa, can certainly have a place. Good luck!
Further reading
Samudrala S. Topical haemostatic agents in surgery: a surgeon’s perspective. AORN J 2008; 88: 2-S11.
Moss R. Management of surgical haemostasis. AORN J 2013. Accessible at: www.aorn.org/Education/Supporting_Documents/Management_of_Surgical_Hemostasis.aspx .
References
- Ker K, Edwards P, Perel P, Shakur H, Roberts I. Effect of tranexamic acid on surgical bleeding: systematic review and cumulative meta-analysis. BMJ 2012: 344: e3054.
- Ranucci M, Isgro G, Soro G, Conti D, De Toffol B. Efficacy and safety of recombinant activated factor VII in major surgical procedures: systematic review and metaanalysis of randomised clinical trials. Arch Surg 2008; 143: 296-304.







Leave a Reply