Eclampsia is defined by the onset of convulsion in the antepartum (after 20 weeks gestation), intrapartum or postpartum period (usually within seven days, but up to a maximum of six weeks postpartum) in association with features of pre-eclampsia. Internationally, eclampsia occurs in 0.6 per cent of all pregnancies and up to 2–3 per cent of women with pre-eclampsia. In Australia, this figure fortunately remains low at 0.1 per cent of all births and 2.6 per cent of those with pre-eclampsia. Eclampsia carries a high risk of mortality (of up to 15 per cent) and morbidity, particularly with cerebrovascular events. The exact pathophysiology of eclampsia is not known, though two models have been proposed based on the role of central hypertension. The first model describes a breakdown of the autoregulatory system of the cerebral circulation, leading to hyper-perfusion, endothelial dysfunction and cerebral pathology such as haemorrhage or oedema.
The second model describes an activation of the autoregulatory system leading to vasoconstriction of cerebral vessels and hypo-perfusion. These pathophysiological factors may result in cerebral irritation and eclampsia. The prodromal importance of hyperreflexia and clonus as signs of upper motor neurone irritation from cerebral oedema is recognised in everyday practice.
Can we predict eclampsia?
A systemic review demonstrated that most women who develop eclampsia experience one or more anteceding symptoms in the preceding few hours (Table 1), though up to 25 per cent may be asymptomatic. It is also important to remember that, rarely, features of pre-eclampsia (hypertension and proteinuria) can be absent in some women prior to eclampsia. There is no general agreement on indication for prophylactic magnesium infusion, though the current literature recommends initiating magnesium sulphate in women who present with:
- Systolic blood pressure (SBP) ≥170mmHg or diastolic blood pressure (DBP) ≥110mmHg with 3+ proteinuria
- SBP ≥150 mmHg or DBP ≥100mmHg with 2+ proteinuria in the presence of at least two antecedal signs or symptoms of eclampsia (Table 1)
- Pre-eclampsia with at least one sign of central nervous system irritability (persistent severe headache, visual disturbance, hyperreflexia with sustained clonus of more than three beats)
- The need for transfer to high dependency care unit for the management of pre-eclampsia
Management of eclampsia
There are four key aims in the management of eclampsia and these are further outlined in Figure 1 (overleaf).
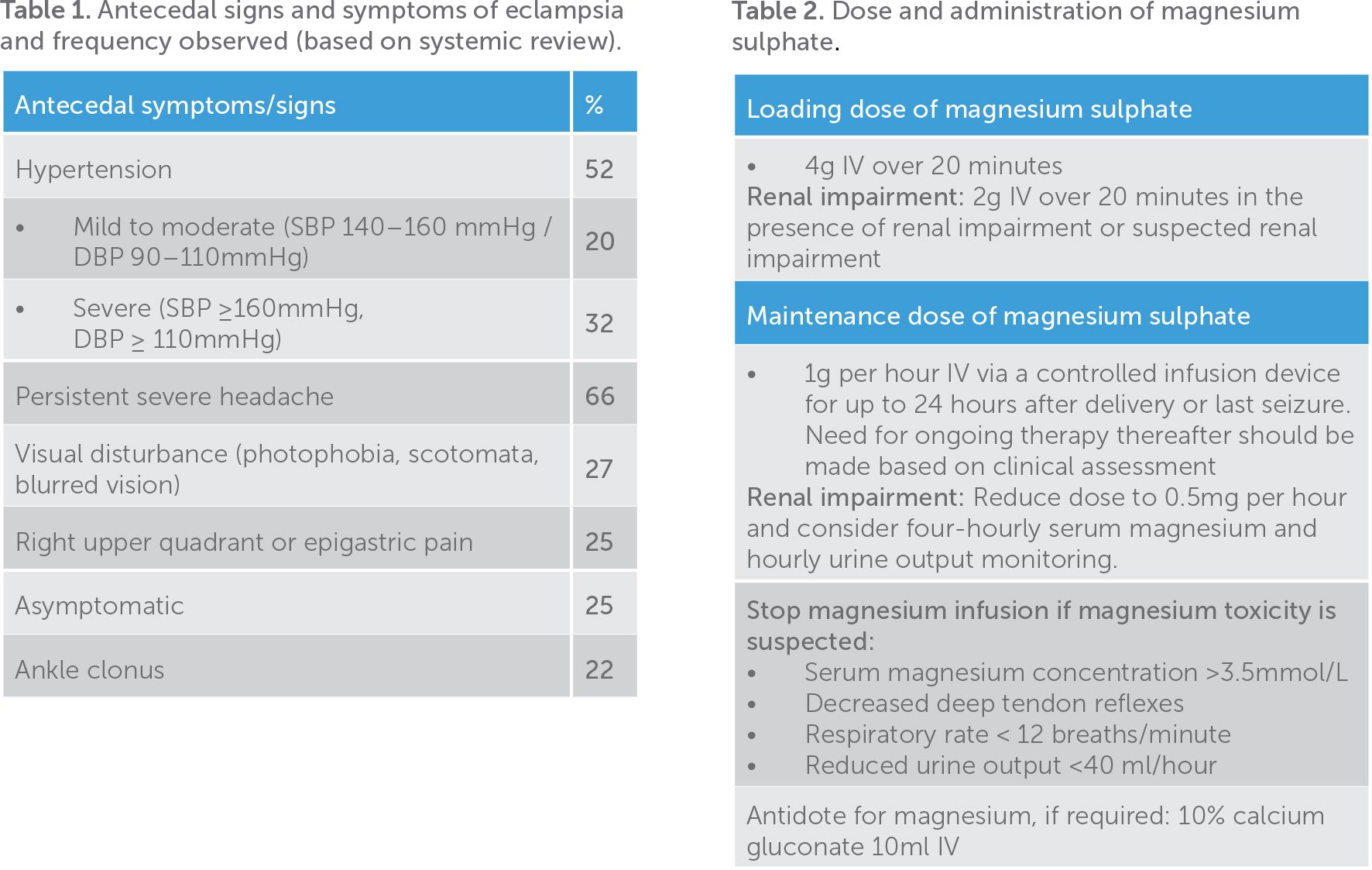
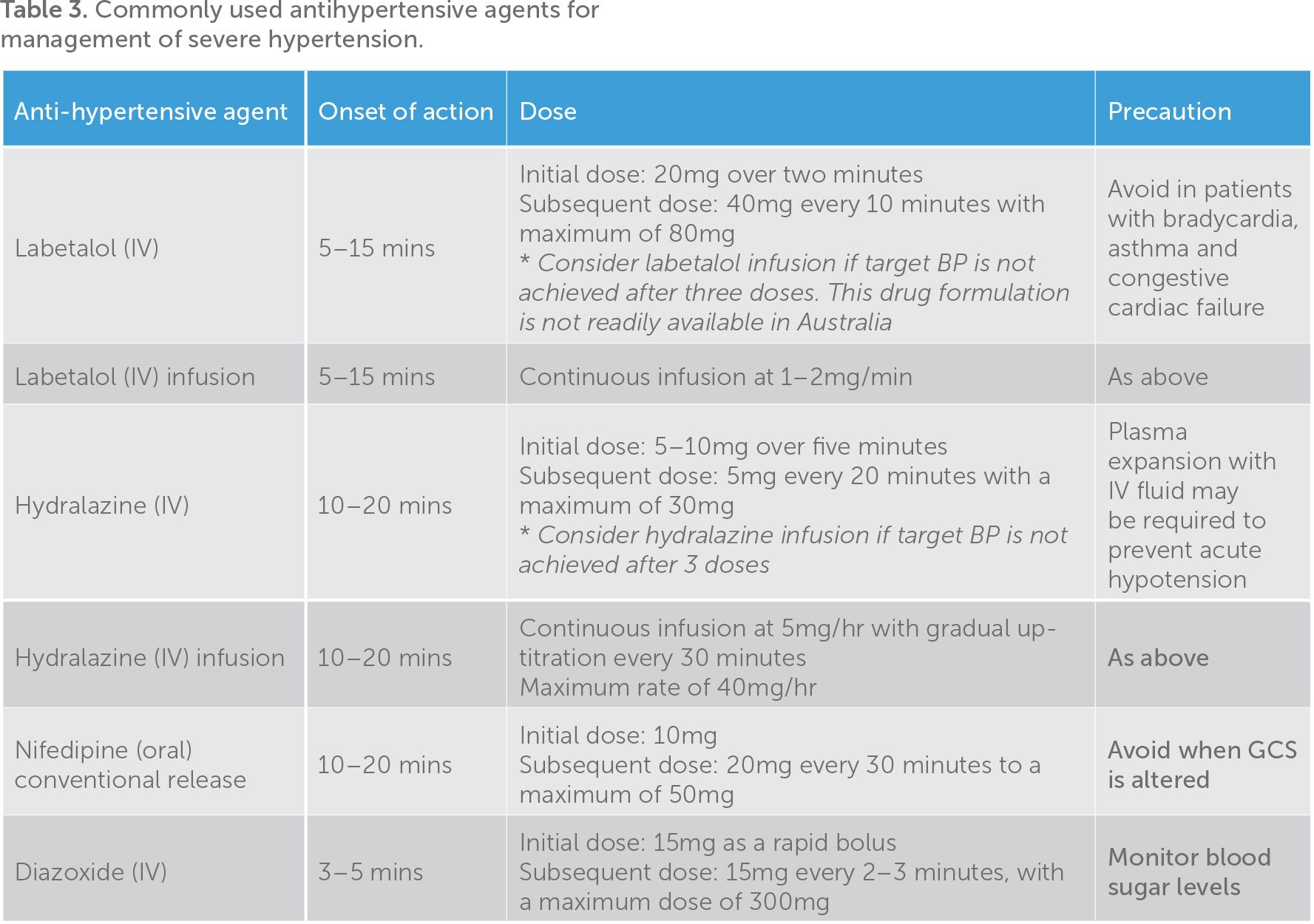
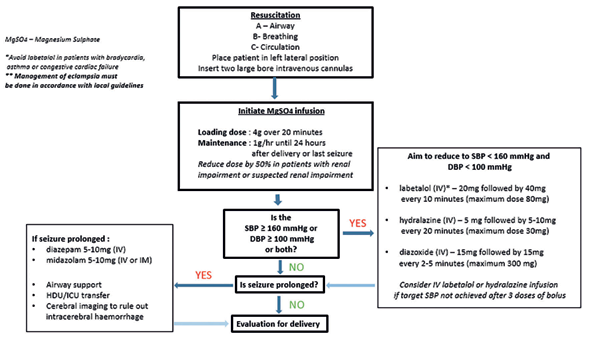
Figure 1. Flowchart summarising management of eclampsia.
Maintenance of maternal airway and prevention of hypoxia
The patient should be placed in a left lateral position to minimise the risk of aspiration with supplemental oxygen at a rate of 8–10L/min administered via a nonrebreather mask. Raise side rails of bed to ensure safety of the patient.
Seizure management and prevention of recurrent seizure
The first eclamptic seizure is usually self-limiting. Intravenous magnesium sulphate should be initiated in patients with eclampsia as a first-line therapy and for prevention of recurrent seizures (Table 2). If intravenous access is not established, magnesium sulphate (4g) or midazolam (5–10mg) can be given intramuscularly in the immediate setting, though access must be subsequently established.
If the seizure is prolonged, benzodiazepines may be required (Figure 1). It is important to exercise a great degree of caution with co-administration of magnesium sulphate and benzodiazepines as this increases the risk of respiratory arrest. It is also important to consider the need to transfer the patient to a high dependency unit and for cerebral imaging in the event of prolonged or recurrent seizure. Magnesium sulphate infusion reduces the risk of recurrent seizure in eclamptic patients by 52 per cent and, for this reason, timely initiation of magnesium sulphate infusion is crucial (Table 2). Magnesium sulphate is renally excreted and a dose reduction is required when renal impairment is present or suspected (urine output <40ml/hr, or a rising serum creatinine concentration). The current recommendation suggests that the infusion should be continued for up to 24 hours after delivery or last seizure, or longer if clinically indicated (ongoing headache, visual changes, hyperreflexia, ankle clonus). An additional bolus of 2g of intravenous magnesium sulphate can be administered if seizure reoccurs while on the magnesium sulphate maintenance infusion. Neurology opinion and cerebral imaging should be obtained if seizure continues to reoccur despite this.
Magnesium sulphate toxicity can lead to a decrease or loss of deep tendon reflexes followed by respiratory depression and ultimately respiratory arrest. The current literature does not recommend routine serum magnesium concentration monitoring in women with normal renal function, though a four-hourly biochemical monitoring is required in those with renal impairment. The current monitoring recommendations for women receiving magnesium sulphate infusion are:
- Hourly deep tendon reflex assessment
(upper limbs if after epidural/spinal anaesthesia) - Hourly urine output monitoring
- Continuous pulse oximetry monitoring
Management of severe hypertension
Acute hypertension management is warranted if SBP ≥160mmHg or DBP ≥100mmHg or both. This is crucial in the prevention of cerebrovascular events, which account for 20–30 per cent of the morbidity and mortality of eclampsia.
It is important to reduce the blood pressure without compromising maternal cerebral perfusion pressure and uteroplacental blood flow. The aim of initial intervention is to reduce SBP to ≤160mmHg and DBP to ≤100mmHg. Intravenous antihypertensive agents are often used in this setting due to the need for rapid onset of action. The agents commonly used are labetalol, hydralazine or diazoxide (Table 3). Selection of the medication should be based on the availability, potential side effects and the clinician’s and caring team’s familiarity. A more detailed description of these medications is provided under the subheading ‘Hypertensive emergencies in pre-eclampsia’.
Evaluation for delivery
While delivery is the definite treatment of eclampsia, eclampsia is not an absolute indication for caesarean delivery. The decision on the mode of delivery is often made based on maternal and fetal stability, gestational age, cervical status and fetal position. The decision and plan for delivery should be made soon after the mother’s airway and seizure activity have been stabilised and every attempt to control blood pressure has been made. This is a decision that involves a multidisciplinary team, including neonatologist, as the risk of ongoing morbidity from the seizure and pre-eclampsia can only be mitigated by delivery.
Hypertensive emergencies in pre-eclampsia
Hypertensive emergency in pre-eclampsia is defined by persistent (more than 15 minutes) of SBP >160mmHg, DBP >100mmHg or both. This can occur in the antenatal, intrapartum or postpartum period. The severity of hypertension is known to correlate with the incidence of cerebrovascular event and, therefore, prompt assessment and management of hypertension is essential. The key factors in management of hypertensive emergencies in pre-eclampsia are:
- Prevention of secondary complications
- Prevention of eclampsia (as detailed in the section ‘Can we predict eclampsia?’)
Prevention of secondary complications
The known complications of hypertensive emergencies in pre-eclampsia are cerebrovascular events (for example, intracerebral haemorrhage or acute pulmonary oedema), placental abruption and maternal arterial dissection. While prompt management of hypertension is required, rapid and marked reduction in SBP can lead to a reduction in uteroplacental blood flow with non-reassuring cardiotocograph tracing, or a maternal hypo-perfusion (for example, cerebral, renal or spinal). Therefore, a target SBP of <160/100mmHg is recommended. Rapid-acting oral and intravenous antihypertensive agents are often used for this purpose (Table 2).
- Intravenous labetalol is a rapid acting betablocker with an onset of action from 5–15 minutes. Labetalol should be avoided in patients with bradycardia, asthma and congestive cardiac failure.
- Intravenous hydralazine is a direct peripheral arteriolar vasodilator with an onset of action from 15–20 minutes. Women who are clinically hypovolemic should receive an intravenous fluid preload of 250–500ml of either sodium chloride 0.9 per cent or Hartmann’s solution to prevent rapid hypotension.
- Intravenous diazoxide is a rapid-acting potent peripheral arteriolar vasodilator with an onset of action from 2–5 minutes. It has been shown to be more effective than intravenous hydralazine in managing resistant hypertension, though its use is often limited by availability and clinician’s familiarity.
- Oral nifedipine (conventional release) is a calcium channel blocker that has been shown to have comparable efficacy as a fast-acting hypertensive agent when compared with intravenous labetalol. However, the use of oral agents should be avoided in patients with altered Glasgow coma score due to the risk of aspiration pneumonia.
Decision on timing of delivery should involve a multidisciplinary team, including obstetricians, physicians and neonatologists. The decision should also take into account the gestational age and maternal and fetal stability.
Further reading
Lowe SA, Bowyer L, Lust K, et al. SOMANZ guidelines for the management of hypertensive disorders of pregnancy 2014. ANZJOG. 2015;55(5):e1-29.
Tranquilli AL, Dekker G, Magee L, et al. The classification, diagnosis and management of the hypertensive disorders of pregnancy: A revised statement from the ISSHP. Pregnancy Hypertension. 2014;4(2):97-104.
The Eclampsia Trial Collaborative Group. Which anticonvulsant for women with eclampsia? Evidence from the Collaborative Eclampsia Trial. Lancet. 1955;345(8963):1455-63.
The Magpie Trial Collaborative Group. Do women with pre-eclampsia, and their babies, benefit from magnesium sulphate? The Magpie Trial: a randomised placebo-controlled trial. Lancet. 2002;359(9321): 1877-90.
Shekhar S, Gupta N, Kirubakaran R, Pareek P. Oral nifedipine versus intravenous labetalol for severe hypertension during pregnancy: a systematic review and meta-analysis. BJOG. 2016;123(1):40-7. Hennessy A, Thornton CE, Makris A, at al. A randomised comparison of hydralazine and mini-bolus diazoxide for hypertensive emergencies in pregnancy: the PIVOT trial. ANZJOG. 2007;47(4):279-85.






Leave a Reply