Postpartum haemorrhage (PPH) is the leading cause of maternal morbidity and mortality worldwide, with WHO estimating that 25 per cent of direct maternal deaths are attributable to PPH.1
Maternal deaths are rare in developed countries such as Australia and New Zealand; however, PPH is not. Estimated to complicate 5–15 per cent of births in Australia,2 it is imperative that all maternity care providers are proficient in managing PPH. Each centre providing care for pregnant women should have well-established protocols that are routinely rehearsed in a multidisciplinary setting.
PPH may be primary, within 24 hours of childbirth, or secondary, from 24 hours to the completion of the puerperium. Blood loss of more than 500ml constitutes a PPH, and more than 1000ml is considered a major PPH.
The third stage of labour
During a normal third stage the uterus contracts, causing separation and expulsion of the placenta and membranes. This contraction of the myometrium compresses the myometrial vessels that have vascularised the placental bed throughout pregnancy. Local decidual haemostatic factors also contribute to haemostasis. At term, uterine artery blood flow is 500–700ml per minute; hence, disruption in either of these mechanisms may lead to rapid haemorrhage.
Retained tissue inhibits the normal ability for the uterus to contract, causing PPH. If unrecognised, this may also cause endometritis and secondary PPH. Birth canal trauma may cause overt and occult blood loss, and women with a thrombophilia, diagnosed or undiagnosed, are also at higher risk of PPH.
The causes of PPH are often summarised as the four Ts: tone, trauma, tissue and thrombin. An additional T has frequently been added to emphasise the utility of an operating theatre in the management of severe PPH.
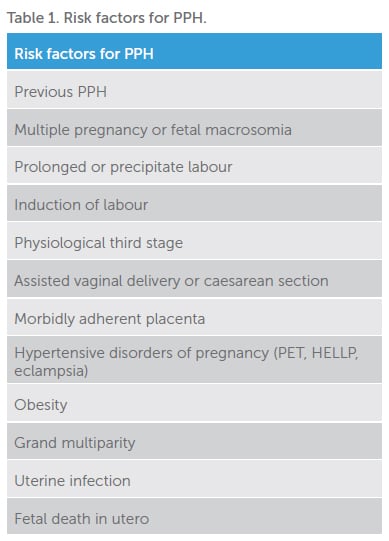
Risk factors
There are multiple identified risk factors for PPH (Table 1), but most occur in women without any. Risk factors may be present antenatally or develop intrapartum; hence, care providers must remain alert and alter management plans accordingly.
Recognition
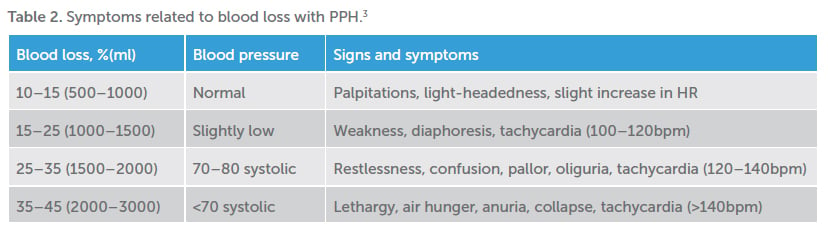
Revealed PPH may be rapid and easily recognised. However, PPH can also occur insidiously, with a slow trickle over many hours, or via occult loss, that is, into a pelvic haematoma, potentially delaying recognition and treatment. This is a particularly dangerous scenario as significant blood loss can lead to coagulopathy and intravascular depletion, making successful resuscitation, once PPH is recognised, all the more difficult.
Due to physiologically expanded circulation, with the healthy parturient carrying a circulating blood volume of approximately 100ml/kg, large volumes of blood may be lost before haemodynamic instability is evident. It is difficult to ‘catch up’ if early losses are not recognised. It is also widely recognised that visual estimation of blood loss may underestimate; hence, weighing is essential to improve accuracy of estimates and guide resuscitative measures.
Management of postpartum haemorrhage
Initial management is two pronged, with simultaneous resuscitation to restore haemodynamic stability and targeted treatment of the underlying cause(s) both paramount to successful outcomes.
General measures when PPH is recognised, include:
- Summon additional assistance and designate roles as appropriate
- Designate a scribe to contemporaneously document events
- Ensure initial and frequent vital signs measured
- Insert two large-bore (16g) IV cannulas
- Send blood for FBE, group and save, coagulation studies
- Insert indwelling catheter with burette for fluid balance
- Initial volume replacement with warmed isotonic crystalloid, that is, CSL or 0.9% NaCl
- Prevent hypothermia, which may exacerbate acidosis and contribute to coagulopathy
Tone
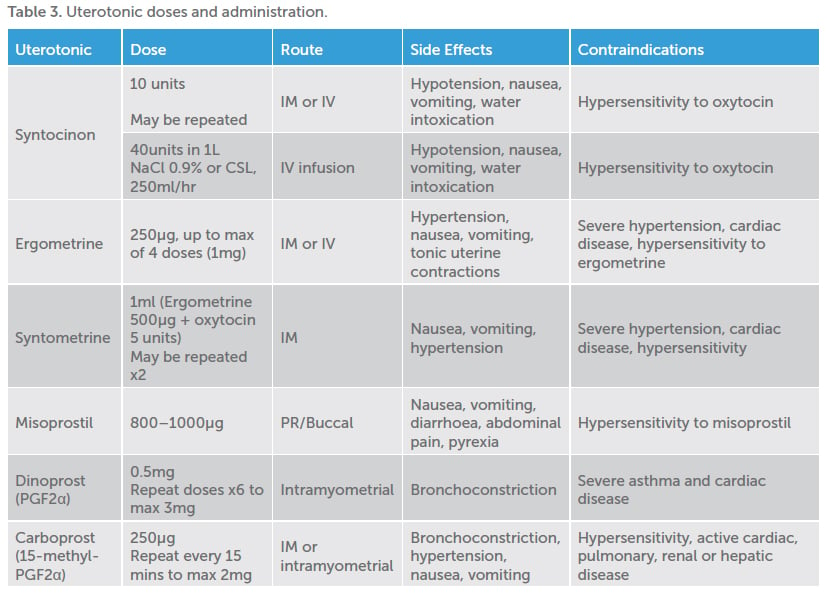
Uterine atony is thought to cause 75–90 per cent of PPH.3 In most instances, atony will respond to first-line uterotonics (Syntocinon, Syntometrine), uterine massage and the insertion of an indwelling catheter. Torrential atonic PPH can be stabilised via bimanual uterine or aortocaval compression while resuscitative efforts commence, assistance obtained and transfer to theatre organised.
Management should include stepwise use of available uterotonics, taking into account individual patient contraindications, with early recourse to definitive surgical management in the unstable patient where medical management is proving ineffective. The discontinuation of local production of dinoprost (PGF2α) has led to the alternate use of carboprost (15-methyl- PGF2α). The reconstitution, dosage and administration of these medications differs; thus, it is important for clinicians to be aware of which they have at their facility, and how to use them.
Trauma
Genital tract trauma is the next most common cause of PPH, with up to 70 per cent of women sustaining perineal tears. High vaginal tears or cervical lacerations may require transfer to the operating room for adequate anaesthesia, lighting and assistance. Third- and fourth-degree tears should be repaired in theatre. Uterine rupture after vaginal birth after caesarean may be suspected in a woman with ongoing blood loss despite a well-contracted fundus in the absence of perineal trauma, or if shoulder-tip pain is present.
Tissue
Retained placenta or membranes inhibit uterine contractions and create a nidus for infection. It is essential that the placenta and membranes be carefully inspected for completeness, with care to exclude a retained cotyledon or succenturiate lobe. A partially separated placenta may cause rapid blood loss. Transfer to an operating theatre is required for provision of anaesthesia, so that complete examination and removal of tissue can be achieved. Morbidly adherent placentation should be considered if a placental-myometrial plane cannot be found at time of manual removal, and additional skilled assistance sought.
Thrombin
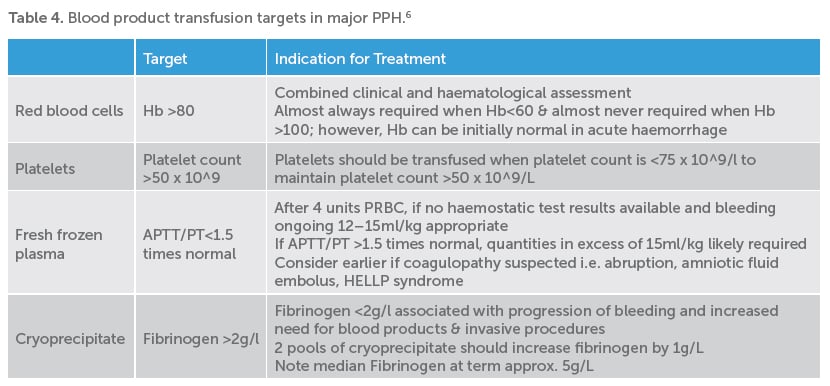
Coagulopathy may be a cause or a result of PPH. Women with a known bleeding diathesis should be antenatally discussed with a haematologist and a clear intrapartum and postpartum plan documented. Early involvement of blood bank and haematology services are essential in cases of major or ongoing PPH.
Point-of-care coagulation testing, including TEG (thromboelastography) and ROTEM (rotational thromboelastometry), is increasingly being used in the management of major PPH. Initial results may be available within as little as five minutes to guide administration of clotting factors and blood products.
Point-of-care testing has been shown to reduce the requirement for blood component therapy when compared to empiric replacement.4
Tranexamic acid (TXA) has a well-defined role in the field of trauma surgery; however, its role in obstetric haemorrhage has been less clearly defined. Initial studies suggested that TXA may reduce total blood loss, fall in haemoglobin and need for blood transfusion;5 however, a Cochrane Review found that trials testing the effectiveness of TXA in obstetric haemorrhage were too small to draw meaningful conclusions.6
Recent publication of the World Maternal Antifibrinolytic Trial (WOMAN) has shed more light on the utility of TXA in obstetric practice.7
WOMAN sought to evaluate the effect of TXA in PPH on mortality and hysterectomy. Eligible women with a clinical diagnosis of PPH received 1g of TXA via slow intravenous (IV) injection after randomisation. If bleeding was ongoing after 30 minutes, or recommenced within 24 hours, a further 1g dose was given. WOMAN demonstrated a significant reduction in deaths from bleeding, particularly when TXA was administered within the first three hours. Importantly, there was no increase in adverse events (such venous thromboembolism) compared with placebo. In light of these findings, one anticipates more widespread use of TXA in obstetric settings to follow.
Theatre
The operating theatre is undoubtedly the appropriate environment to manage any major PPH. Early recourse to definitive surgical management should be considered in the unstable woman where medical management is ineffective. The UK Confidential Enquiry into Maternal and Child Health repeatedly cites delay or failure to perform hysterectomy as an avoidable cause of maternal death from PPH.8
Fertility-preserving management options in theatre include:
- Intrauterine balloon tamponade such as Bakri Balloon. Using warmed fluid to fill the balloon is advantageous as it accelerates the clotting cascade
- Uterine compression sutures, such as B-Lynch suture, using an absorbable monofilament suture, such as Monocryl
- Arterial ligation of uterine or internal iliac arteries greatly reduces uterine blood flow. Care to avoid ureters and other vessels when operating on the pelvic sidewall is necessary
- Arterial embolisation via interventional radiology where facilities are available
A consultant obstetrician should be involved in all decisions regarding peripartum hysterectomy, ideally in consultation with another senior consultant.
Outcomes and follow-up
Appropriate clinical monitoring in the postpartum period is essential after PPH as there are multiple possible sequelae that may carry significant morbidity, such as acute renal failure, Sheehan syndrome, ileus and venous thromboembolism.
The experience of PPH may be traumatic for mothers and their families. Those involved in managing the PPH should debrief the woman and family as required, with low thresholds to involve appropriate specialist perinatal mental health support. The provision of a follow-up outpatient appointment to answer any further questions, follow-up investigations and to plan future pregnancies is invaluable.
References
- World Health Organisation. WHO recommendations for the prevention and treatment of postpartum haemorrhage. 2012. Geneva. Available from: http://apps.who.int/iris/bitstream/10665/75411/1/9789241548502_eng.pdf?ua=1.
- Henry A, Birch M R, Sullivan E A, et al. Primary PPH in an Australian tertiary hospital: a case-control study. ANZJOG. 2005;45:233-36.
- Rani PR, Begum J. Recent advances in the Management of Major Postpartum Haemorrhage. J Clin Diagn Res. 2017;11(2): QE01-5.
- Mallaiah S, Barclay I, Harrod C, et al. Introduction of an algorithm for ROTEM-guided fibrinogen concentrate administration in major obstetric haemorrhage. Anaesthesia. 2015;70:166-75.
- Duclo-Bouthors AS, Jude B, Duhamel A, et al.; The EXADELI Study Group. High-dose tranexamic acid reduces blood loss in postpartum haemorrhage. Crit Care. 2011;15:R117.
- Mousa HA, Blum J, Abou El Senoun G, et al. Treatment for primary postpartum haemorrhage. Cochrane Database Syst Rev. 2014;(2):CD003249.
- Shakur H, Roberts I, Bukola F, et al. Effect of early tranexamic acid administration on mortality, hysterectomy, and other morbidities in women with post-partum haemorrhage (WOMAN): an international, randomised, double-blind, placebo-controlled trial. Lancet. 2017. http://dx.doi.org/10.1016/S0140-6736(17)30638-4.
- Lewis, G (ed). The Confidential Enquiry into Maternal and Child Health (CEMACH). Why Mothers Die 2000–2002. The Sixth Report on Confidential Enquiries into Maternal Deaths in the United Kingdom. 2004. London: CEMACH.






Leave a Reply