For the broader O&G Magazine readership, balanced answers to those curly-yet-common questions in obstetrics and gynaecology.
Heavy menstrual bleeding (HMB) is one of the most common gynaecological symptoms for which women seek medical care.1 2 One in 20 women suffer from HMB.3 4 Several endometrial techniques are available for treatment, provided that intracavity abnormalities are absent. Modern endometrial ablation devices are effective in the treatment of HMB with high patient satisfaction rates.5
What is the difference between different generations of endometrial ablation devices?
Endometrial ablation (EA) refers to a series of blind techniques originating in the 19th century, which used various sources to achieve thermal destruction of the endometrium. The late 20th century brought an important paradigm shift when a rod lens hysteroscope was used together with an energy source permitting EA under direct visualisation. However, the early hysteroscopic resectoscopic techniques required training owing to their complexity and more user-friendly techniques with reported improved safety are now available.6
How does NovaSure ablation work?
The NovaSure system comprises of a disposable ablation device, a radiofrequency generator (RF), a suction line desiccant and a carbon dioxide canister.7 The ablation device is a conformable, bipolar electrode array housed within a protective sheath and mounted on an expandable frame (Figure 1). It also contains an intrauterine measuring system that determines the uterine cavity width (cornua–cornua distance). When the ablation device is seated correctly within the cavity, the sheath is withdrawn into the endocervical canal to protect the endocervix from thermal injury.8 Using the uterine width and length, the RF controller calculates the appropriate power output to ensure complete ablation of the endometrium. During ablation, the tissue impedance is continuously monitored and the procedure terminates once a level of 50 ohms is achieved. Actual treatment may vary up to 120 seconds to accommodate different uterine dimensions and endometrial thickness. Carbon dioxide is delivered into the uterine cavity at a safe flow rate and pressure through the central lumen of the ablation device. When 50mmHg is achieved for four seconds, uterine integrity is confirmed. If this intrauterine pressure is not achieved due to a perforation or a leak through the cervix, the procedure cannot be performed.9 A vacuum pump in the RF controller brings the endometrial lining into contact with the electrode array and simultaneously removes byproducts of ablation from the uterine cavity. Compared to other ablation techniques, NovaSure procedure has the shortest treatment time, requires no uterine pretreatment and can be performed at any time during the menstrual cycle.10
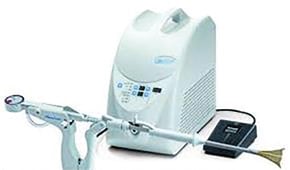
Figure 1. NovaSure ablation device.
What are the success rates?
Satisfaction rates for NovaSure ablation are high, ranging from 77–96 per cent, amenorrhoea occurs in 14–70 per cent and quality of life (including pleasure and discomfort) scores from women with HMB are substantially improved.11 12 13 Treatment failure is defined as; hysterectomy for any benign indication, repeat ablation within 36 months of the procedure, synechiolysis or treatment with gonadotrophin-releasing hormone for post-ablation pain or bleeding.
Pre-operative predictors
A paper published in 2014 demonstrated that women with a history of tubal ligation were more likely to experience treatment failure compared to women without a history of tubal ligation (16.4 per cent compared with 9.0 per cent, P=0.0008).14 Women with preoperative dysmenorrhea, pelvic pain or obesity were more likely to experience treatment failure after ablation (21.8 per cent compared with 10.7 per cent, P =0.002 and 16.7 per cent compares with 9.8 per cent P =0.003 respectively).15 Tubal ligation is a well-recognised risk factor for hysterectomy after endometrial ablation, secondary to post-ablation syndrome, which has an incidence of 6–8 per cent and usually develops 2–3 years after endometrial ablation. Other predictors of failure are age younger than 45 years, adjusted hazard ratio (aHR) 2.6, P=0.008 and parity >5 aHR 1.3 – 6.0, P <0.001 and finally, ultrasound features suggestive of adenomyosis aHR 1.5. P =.003.16
Intraoperative predictors
Intraoperative predictors of failure are uterine sounding length >10.5cm (aHR 2.58), uterine cavity length >6cm (aHR 2.06), uterine width >4.5cm (aHR 2.06) and procedure time <93 seconds (aHR 2.61).13 Of interest, previous caesarean section is not a pre-operative or intra-operative predictor for failure of ablation. The risk of bowel injury at the time of NovaSure ablation is 1:5000.17
What are the short- and long-term complications?
Pregnancy-related complications
Endometrial ablation is not considered a form of contraception. Pregnancy has been reported to occur in 0.7 per cent of women who have undergone a tubal ligation.18 Pregnancy has been reported as early as five weeks and as late as 12 years postoperatively. The chances of pregnancy occurring after endometrial ablation and tubal ligation is estimated to be at 0.002 per cent or one in 50,000. Pregnancy has also been reported in amenorrhoeic women.19 There is no significant increase over the baseline miscarriage rate; however, the risk of ectopic pregnancy is approximately 2 per cent.20
Pain-related obstructed menses
When energy is applied to the endometrium, tissue necrosis and inflammation can result in uterine contracture and intrauterine scarring. This has been demonstrated with both resectoscopic endometrial ablation and the newer devices, such as NovaSure ablation.21 Persistent endometrium after endometrial ablation is common as evidenced by clinical, imaging, and histologic studies. Randomised clinical studies have shown that amenorrhoea rates are generally less than 50 per cent, suggesting persistent endometrial glands after endometrial ablation. Contracture and scarring at the fundus can lead to obstructed degrees of menses. This can manifest as haematometra within the body of the uterine cavity (central haematometra) or at the cornual region. In cases of post-ablation tubal sterilisation syndrome, symptom relief has been reported after removal of the tubes.22 Central haematometra is most likely to occur when the cervical canal is damaged at the time of endometrial ablation and has an incidence of 1–3 per cent.23 Central haematometra can usually be treated with cervical dilatation; however, in some cases, hysteroscopic adhesiolysis may be required.
Failure to control menses
A discussion centred around patient expectations is an important aspect of pre-operative counselling. Most women who undergo endometrial ablation will not experience amenorrhoea, yet 85 per cent will be satisfied at the one-year mark.24 The evidence for long-term failure of endometrial ablation may be examined by a 4–5 year re-operation rate of 18–38 per cent.25For patients who are not satisfied with endometrial ablation, most case series report hysterectomy as the next step. Repeat endometrial ablation was not evaluated as part of the FDA for NovaSure ablation and should be considered off-label use.
What if she has symptoms?
Evaluating the uterine cavity and cancer risk after NovaSure ablation
The risk of endometrial cancer increases by approximately 2–3-fold between ages 50 and 70 years; it is possible that women who have undergone endometrial ablation in the last two decades are now entering the age group at risk for endometrial cancer.20 These patients should be evaluated with caution when abnormal uterine bleeding occurs and a diagnosis of endometrial cancer should be taken into consideration. Patients with bleeding after an endometrial ablation create a diagnostic dilemma because the basic tools for evaluation may fail to yield substantial results. In addition, the anatomical distortion can alter features at ultrasonography, making a noninvasive diagnosis challenging.26 However, a systematic review found that even though endometrial sampling and investigation of abnormal uterine bleeding may be difficult to perform, it remains feasible.27 In only two cases (11.8 per cent) a preoperative diagnosis of endometrial biopsy was not successful at presentation because of cervical stenosis and intrauterine adhesions.28 Therefore, in most cases, preoperative diagnosis of endometrial cancer or hyperplasia is possible. This is contrary to concerns that diagnosis may be delayed or difficult to achieve. If unable to perform pipelle because of cervical stenosis, hysteroscopy should be attempted given that evidence shows that it is feasible. Using a smaller hysteroscope, such as a 3mm scope, with vaginal estrogen for a few weeks prior to procedure (for postmenopausal patients with vaginal atrophy) and 400mμg buccal misoprostol 30 minutes pre-operatively has been found to be helpful at our endosurgery unit.
Endometrial ablation is not a treatment for endometrial hyperplasia or cancer and may interfere with subsequent evaluation of the endometrium. The safety of endometrial ablation has not been well studied in women who are at increased risk of developing endometrial cancer. These risks include nulliparity, chronic anovulation, obesity, diabetes mellitus, tamoxifen therapy and hereditary nonpolyposis colorectal cancer.29 An early concern about the safety of endometrial ablation was the fear that it may delay the diagnosis of a subsequent endometrial carcinoma. However, it appears that in most instances the intrauterine cavity is not completely destroyed, thus allowing degrees of bleeding from the retained endometrium. A systematic review, published in 2011, examining endometrial cancers diagnosed five years after NovaSure ablation, confirmed that most women with post-ablation endometrial cancer present with abnormal uterine bleeding and pain.30 Over 75 per cent of the women with post-ablation endometrial cancer were diagnosed with stage I endometrial cancer. This is in keeping with the typical presentation of endometrial cancer in women without a history of endometrial ablation. Furthermore, most women with endometrial cancer post ablation remained able to have the endometrium sampled by pipelle or hysteroscopy.31
Conclusion
In the 13 years since the NovaSure procedure was approved by the FDA, significant data has been generated that provides a good safety profile, low re-intervention rates and high patient satisfaction rates.
References
- Oehler MK, Rees MC. Menorrhagia: an update. Acta Obstet Gynecol Scand. 2003;82:405-22.
- Liu Z, Doan QV, Blumenthal P, Dubois RW. A systematic review evaluating health-related quality of life, work impairment, and health care costs and utilization in abnormal uterine bleeding. Value Health. 2007;10:173-82.
- Vessey MP, Villard-Mackintosh L, McPherson K, et al. The epidemiology of hysterectomy: findings in a large cohort study. BJOG. 1992;99:402-7.
Coulter A, McPherson K, Vessey MP. Do British women undergo too many or too few hysterectomies? Soc Sci Med. 1988;27:987-94.- Bongers MY, Bourdrez P, Mol BW, et al. Randomized controlled trial of bipolar radio-frequency endometrial ablation and balloon endometrial ablation. BJOG. 2004;111:1095-102.
- Morris W, Aarthi C, Arthur M, Vance M. Late-onset endometrial ablation failure – Etiology, Treatment and Prevention. J Minim Invasive Gynecol. 2015;22:323-31.
- US Food and Drug Administration, Center for Devices and Radiological Health. P020031 NovaSure impedence controlled endometrial ablation system approval letter. September 28, 2001.
- US Food and Drug Administration, Center for Devices and Radiological Health. P020031 NovaSure impedence controlled endometrial ablation system approval letter. September 28, 2001.
- US Food and Drug Administration, Center for Devices and Radiological Health. P020031 NovaSure impedence controlled endometrial ablation system approval letter. September 28, 2001.
- Cooper J, Gimpelson R, Laberg P, et al. A randomized, multicenter trial of safety and efficacy of the NovaSure system in the treatment of menorrhagia. J Am Assoc Gynecol Laparosco. 2002;9,418-28.
- Lethaby A, Hickey M. Endometrial destruction techniques for heavy menstrual bleeding: a Cochrane review. Human Reprod. 2002;17(11):2795.
- Herman M, Penninx J, Mol B, Bongers M. Ten year follow up of a randomized controlled trial comparing bipolar endometrial ablation with balloon ablation for heavy menstrual bleeding. BJOG. 120;(8):966-70.
- Maher PJ, Hill DJ. Transcervical endometrial resection for abnormal uterine bleeding–report of 100 cases and review of the literature. ANZJOG. 1990;30(4):357-60.
- Smithing KR, Savella G, Raker CA, et al. Preoperative uterine bleeding pattern and risk of endometrial ablation failure. Am J Obstet Gynecol. 2014;211:556.
- Smithing KR, Savella G, Raker CA, et al. Preoperative uterine bleeding pattern and risk of endometrial ablation failure. Am J Obstet Gynecol. 2014;211:556.
- El Nashar SA, Hopkins MR, Creedon DJ, et al. Prediction of treatment outcomes after global endometrial ablation. Obstet Gynecol. 2009;113:97-106.
- Adkins RT, Phillip L, Bressman, et al. Radiofrequency endometrial ablation in patient with a history of low transverse cesarean delivery. J Minim Invasive Gynecol. 2013;20:840-52.
- Pugh CP, Crane JM, Hogan TG. Successful planned pregnancy following endometrial ablation with YAG laser. J Am Assoc Gynecol Laparosc. 2000;7:391-4.
- Palep-Singh M, Angala P, Seela R, Mathur R. Impact of microwave endometrial ablation in the management of subsequent unplanned pregnancy. J Minim Invasive Gynecol. 2007;14:365-6.
- Sharp HT, Endometrial Ablation: postoperative complications. Am J Obstet Gynecol. 2012;207(4):242-7.
- Sharp HT, Endometrial Ablation: postoperative complications. Am J Obstet Gynecol. 2012;207(4):242-7.
- Sherif AM, Shazly SA, Abimbola O, et al. Intraoperative predictors of long-term outcoes after radiofrequency endometrial ablation. J Minim Invasive Gynecol. 2016;23(4):582-9
- Hill DJ. Haematometria – a complication of endometrial ablation/resection. J Am Assoc Gynecol Laparosc. 1994;1:S14.
- Kleijn J, Engels R, Bourdrez P, et al. Five year follow up of a randomized controlled trial comparing NovaSure and Thermachoice endometrial ablation. BJOG. 2008;115:193-8.
- El Nashar SA, Hopkins MR, Creedon DJ, et al. Prediction of treatment outcomes after global endometrial ablation. Obstet Gynecol. 2009;113:97-106.
- Perrella RR, McLucas B, Ragavendra N, et al. Sonographic findings after surgical ablation of the endometrium. AJR Am J Roentgenol. 1992;159(6):1239-41.
- AlHilli MM, Hopkins MR, Famuyide AO. Endometrial cancer after endometrial ablation: systematic review of medical literature. J Minim Invasive Gynecol. 2011;18(3):393-400.
- AlHilli MM, Hopkins MR, Famuyide AO. Endometrial cancer after endometrial ablation: systematic review of medical literature. J Minim Invasive Gynecol. 2011;18(3):393-400.
- Sharp HT, Endometrial Ablation: postoperative complications. Am J Obstet Gynecol. 2012;207(4):242-7.
- AlHilli MM, Hopkins MR, Famuyide AO. Endometrial cancer after endometrial ablation: systematic review of medical literature. J Minim Invasive Gynecol. 2011;18(3):393-400.
- AlHilli MM, Hopkins MR, Famuyide AO. Endometrial cancer after endometrial ablation: systematic review of medical literature. J Minim Invasive Gynecol. 2011;18(3):393-400.







Hi, I had a nova shore ablation when I was 28 due to heavy periods after having my tubes tied following my third child. I experienced a lighter period for a couple of months however 4-5 years on at 33 yrs old it has become extremely painful and irregular. I have had an ultrasound recently which shows a bulky uterus and obviously the lining has fully regrowth. I was told that the ablation was a short term fix so I assume the next Stephie hysterectomy. I am now ok with that.i was told originally that abaltion would only maybe work for 5 years at best and that seems to be the case in my situation.