Polycystic ovary syndrome (PCOS) is the most common endocrine disorder in women of reproductive age; the exact incidence is dependent on diagnostic criteria used, but ranges from six to 20 per cent of the female population.1 Since 2003, the diagnosis has been based on the Rotterdam Criteria encompassing the three facets of the syndrome: sonographic appearance of polycystic ovaries; clinical or biochemical evidence of hyperandrogenism; and oligomenorrhoea with presence of two of the three being diagnostic.2
Recent advances in ultrasound technology and a greater appreciation of androgenic features in certain ethnic groups and adolescents has generated discussion regarding the overdiagnosis of the condition and a move to making diagnostic criteria more exclusive. In 2013, the ultrasound criteria for the description of a polycystic ovary morphology were revised upwards to more than 25 follicles per ovary and an ovarian volume of greater than 10mL.3 The most relevant phenotypical features of the PCOS diagnosis are being explored, with the aim to guide practitioners in identifying patients most at risk of the metabolic sequelae of PCOS. Patients with phenotypes A and B in the following table, therefore, the patients who have evidence of hyperandrogenism with ovulatory disorder as a feature of the disease, appear to be more at risk of metabolic complications than those with phenotype C and D.4
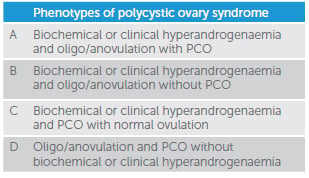
Table 1. Phenotypes of polycystic ovary syndrome
The diagnosis of PCOS in adolescence is even more controversial, as many of the PCOS diagnostic criteria may be physiologically normal for puberty: oligomenorrhoea, cystic acne and PCO morphology, for example. Hirsutism, moderate inflammatory acne which is unresponsive to topical treatment, or persistent menstrual disturbance two years after menarche, raises concerns for PCOS in an adolescent patient, more so than adult criteria for diagnosis.5 Of particular relevance for contraceptive discussions with adolescents is that, despite the fact their menstrual cycle may be very erratic, protracted and unpredictable, evidence suggests the majority are still ovulatory.6
Investigation and management of the metabolic complications of PCOS
Why is it important to diagnose a woman with PCOS? We know patients with PCOS are more likely to develop metabolic complications, including: impaired glucose tolerance (IGT); gestational diabetes and type 2 diabetes mellitus (T2DM); visceral adiposity; dyslipidaemia; vascular endothelial dysfunction; and have a pro-thrombotic state.7 Identifying these women, initiating investigation, and if necessary, lifestyle advice and treatment are integral parts of the management of PCOS.
Obesity
Obesity is not a diagnostic feature of PCOS, but women with PCOS are twice as likely to be obese than women without PCOS. Many theories as to the association of PCOS and obesity have been explored. Women with PCOS, even lean women, have lower rates of lipolytic activity and larger adipocytes that respond at slower rates to lipolysis than matched non-PCOS controls.8 Increased androgen levels have been shown to increase appetite and also reduce impulse control, resulting in larger energy consumption in women with PCOS. Gastrointestinal satiety peptides have been shown to be lower in women with PCOS than age and weight matched controls, further supporting the reduction in appetite control in women with PCOS.
The severity of all metabolic risk factors associated with PCOS are exaggerated in women with PCOS who are also overweight or obese. Obesity causes a chronic low-grade inflammation, increasing cardiovascular risk and insulin resistance. Adipose deposits are more likely to be visceral in women with PCOS based on anthropometric measurements of women with and without PCOS, which further increases cardiovascular risk. Weight loss and maintenance of weight within a healthy weight range is the hallmark of first-line treatment for PCOS. Lifestyle factors should be addressed with diet and exercise programs. No specific diet regimes have been shown to be significantly superior in women with PCOS, but high protein diets seem to be better tolerated with associated greater satiety.
If lifestyle factors have failed, bariatric surgery should be considered in women with a BMI greater than 40kg/m2, or greater than 35kg/m2 with additional evidence of metabolic disease. Bariatric surgery has been shown to be successful in reducing weight, PCOS symptoms and metabolic complications of the disorder. Metformin can also be employed and has been demonstrated to lead to reductions in BMI, in association with diet and exercise at a greater rate than lifestyle modifications alone. Other pharmacotherapies are showing promise although there is less safety data, particularly in women who wish to conceive. The glucagon-like peptide-1 (GLP-1) analogue, liraglutide, is a subcuticular injection that reduces appetite. Liraglutide has been successful in reducing bodyweight and fasting plasma glucose levels in obese PCOS patients, in combination with metformin, at greater rates than with metformin alone. Orlistat, a gastric and pancreatic lipase inhibitor that inhibits fat absorption, has better safety data than most weight loss medications on the market. Orlistat has good data demonstrating weight loss, an improved metabolic profile and increased ovulation rates in obese women with PCOS.
Hyperinsulinaemia
Women with PCOS are more likely to be insulin resistant, independent of their BMI, with the incidence of gestational diabetes, impaired glucose tolerance and type 2 diabetes mellitus all being increased in women with PCOS when compared with age and BMI matched controls.9 There is evidence of impaired glucose tolerance in 30 to 75 per cent of women with PCOS and a normal range BMI, while this increases to 95 per cent in PCOS patients who are overweight or obese.9 Screening for insulin resistance in women with PCOS is a crucial part of management. Fasting insulin or fasting glucose levels lack sensitivity and result in underdiagnosis in this group of patients. The recommended test is a two- hour oral glucose tolerance test, repeated every
two years.10
The primary treatment for insulin resistance is weight loss, with associated increase in physical activity. This also improves fertility, as well as pregnancy and mental health outcomes. Even if weight loss is not achieved, increased levels of physical activity will improve insulin resistance, as well as altering fat distribution and reducing overall cardiovascular risk.When this fails, medical treatment, often metformin as a first-line agent, can be commenced.
Dyslipidaemia
Dyslipidaemia, like many metabolic complications of PCOS, is more pronounced in women with PCOS and increased BMI. However, even PCOS patients in the normal weight range are at increased risk of dyslipidaemia compared with age and weight matched controls. PCOS patients have lower levels of protective high density lipoprotein (HDL) cholesterol and higher levels of particularly triglycerides, but also very low density lipoprotein (VLDL) and total cholesterol.11
Specific testing for dyslipidaemia for all women with PCOS is not recommended. However, an appreciation of the increased risk of the condition in women with PCOS and thus incorporating the diagnosis into the decision for testing and subsequent treatment with statin therapy, should be made in conjunction with a review of other cardiovascular risk factors that are present.
Cardiovascular disease
Although there is little evidence to support an increased rate of cardiovascular events in women with PCOS, surrogate markers of cardiovascular disease and significant risk factors for cardiovascular morbidity and mortality are increased in women with PCOS. Coronary artery calcification and carotid artery intima-media thickness have both been shown to be more prevalent in women with PCOS. Interestingly, a study looking at the presence of aortic plaque found no significant increased rate in women with PCOS, but the majority of these women were diagnosed based on PCO morphology and oligomenorrhoea only, with no hyperandrogenism features,12 further suggesting the PCOS phenotypes A and B are at greater risk of metabolic disease. An appreciation of these findings should further guide practitioners to recommend weight loss, regular exercise and smoking cessation, as well as reducing other modifiable cardiovascular risk factors in women with PCOS.
Thromboembolism
Women with PCOS are more likely to develop venous thromboembolism (VTE) irrespective of age and BMI.13 Higher levels of fibrinogen, as well as increased rates of endothelial and platelet dysfunction, are thought to be the mechanisms placing PCOS patients at higher risk of VTE.14 Populations studies have put the increased risk at 1.5 times the background risk for a patient’s age and BMI. This is increased to twice the background risk for PCOS patients on the combined oral contraceptive pill (COCP), when compared with non-PCOS patients also on the COCP.15 As the COCP is a commonly prescribed therapy for women with PCOS for treatment of hyperandrogenism, cycle regularity and endometrial protection, practitioners should be aware of the added risk above the population rate of VTE in PCOS women. Further review of other risk factors for VTE should be made when deciding to prescribe the COCP in these women.
PCOS is a metabolic disorder and is associated with an increased risk of multiple metabolic complications. Patients should be made aware of this with diagnosis and encouraged to achieve and maintain a healthy weight range while participating in regular exercise. Impaired glucose tolerance is the only metabolic complication that should be routinely screened for with an alternate yearly oral glucose tolerance test (OGTT). Other metabolic complications should be screened for and treated on a case by case basis, dependent on other cardiovascular risks.
References
- Hart R, Hickey M, Franks S. Definitions, prevalence and symptoms of polycystic ovaries and polycystic ovary syndrome. Best Pract Res Clin Obstet Gynaecol. 2004 Oct;18(5):671-83.
- Rotterdam ESHRE/ASRM-Sponsored PCOS Consensus Workshop Group. Revised 2003 consensus on diagnostic criteria and long-term health related risks related to polycystic ovary syndrome. Fertil Steril 2004; 81:19-25
- Dewailly D, et al. Definition and significance of polycystic ovarian morphology: a task force report from the Androgen Excess and Polycystic Ovary Syndrome Society. Hum Reproduction Update 2014; 20:334-352.
- Moran L, Teede H. Metabolic feature of the reproductive phenotypes of polycystic ovary syndrome. Human Reproduction Update 2009; 15:477-488.
- Ibanez, et al. An International Consortium Update: Pathophysiology, Diagnosis, and Treatment of Polycystic Ovarian Syndrome in Adolesence. Horm Res Paediatr 2017
- Doherty D, Atkinson H, Hickey M, Norman R, Hart R. The majority of irregular menstrual cycles in adolescence are ovulatory: results of a prospective study. NCBI 2017. Hyperlink: www.ncbi.nlm.nih.gov/pubmed/?term=hart+and+doherty+and+ovulatory
- Hart R, Doherty DA. The potential implications of a PCOS diagnosis on a woman’s long-term health using data linkage. J Clin Endocrinol Metab. 2015;100(3):911-9.
- Faulds G, et al. Mechanisms behind lipolytic catecholamine resistance of subcutaneous fat cells in the polycystic ovary syndrome. J Clinical Endocrinol Metabolism 2003; 88:2269-73.
- Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BG, Wong JL, Norman RJ, Costello MF. Guideline Development Groups.Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust. 2011; 195(6):S65-112.
- Teede HJ, Misso ML, Deeks AA, Moran LJ, Stuckey BG, Wong JL, Norman RJ, Costello MF. Guideline Development Groups.Assessment and management of polycystic ovary syndrome: summary of an evidence-based guideline. Med J Aust. 2011; 195(6):S65-112.
- Diamanti-Kandarakis E, et al. Pathophysiology and types of dyslipidaemia in PCOS. Trends Endocrinol. Metab. 2007; 18:280-285.
- Chang AY, Ayers C, Minhajuddin A, et al. Polycystic ovarian syndrome and subclinical atherosclerosis among women of reproductive age in the Dallas Heart Study. Clin Endocrinol 2011; 74:89-96.
- Moran KJ, et al. A comprehensive assessment of endothelial function in overweight women with and without polycystic ovary syndrome. Clin Science 2009; 116:761-70.
- Moran KJ, et al. A comprehensive assessment of endothelial function in overweight women with and without polycystic ovary syndrome. Clin Science 2009; 116:761-70.
- Bird ST, et al. Risk of venous thromboembolism in women with polycystic ovary syndrome: a population-based matched cohort analysis. Canadian Medical Association Journal 2013;185(2):115-120.







Leave a Reply