On 1 December 2017, the Australian National Cervical Screening Program (NSCP) moved to primary screening, with human papillomavirus (HPV) screened at five-yearly intervals for women aged 25–74 years.
Other changes in the NSCP include:
- Standardised screening reports
Results are sorted into one of three categories. All cervical screening test (CST) reports are colour-coded: green for low-risk and a repeat test in five years; orange for intermediate-risk, with recommendation to repeat the test in 12 months; and red for higher-risk, where the woman should go to colposcopy. - Self-collection service
The NCSP offers the potential for self-collection for under-screened and never-screened women. Some laboratories have been accredited by the Therapeutic Goods Administration (TGA) and the National Association of Testing Authorities (NATA) to provide this service. Check with your laboratory service on availability. - National Cancer Screening Register
A National Cancer Screening Register (NCSR) is now live and operational. The register will create a national record for each Australian participating in cervical screening. To access the register to check on your patients’ screening history, or to update their personal details, please call 1800 627 701. - Colposcopy reporting to the register
Colposcopists are now required to notify prescribed cervical screening information to the Chief Medical Officer within 14 days of each colposcopic episode. You can order forms from: www.cancerscreening.gov.au/cervicalforms.
These changes mark an enormous shift in the NCSP for Australia, and one that is expected to result in higher detection rates, with longer screening intervals and better outcomes for women. In combination with the HPV vaccination program, we expect that the rate of cervical cancer will fall to extremely low levels in Australia.
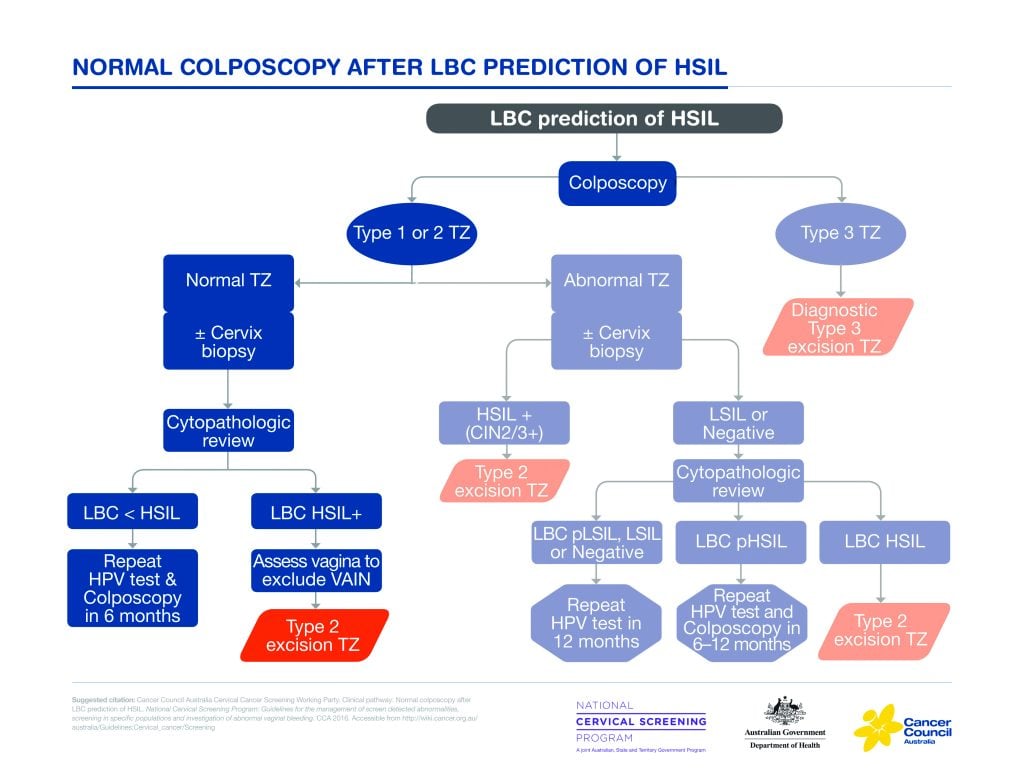
Figure 1. Normal colposcopy after LBC prediction of HSIL.
All information about the updated program is available in a comprehensive 300-page guideline on a Wiki platform.1 This resource provides background information on all of the topics, as well as discussion and evidence behind the recommendations. These guidelines can be easily accessed and contain 156 recommendations. See Table 1 for an overview.
| Table 1. Cancer Guidelines Wiki overview of recommendations2 |
Evidence-based recommendation |
| Formulated based on a systematic review of quality evidence and graded according to a National Health and Medical Research Council-approved method |
Medical Services Advisory Committee (MSAC) evidence-based recommendation |
| Formulated after a systematic review of the evidence, indicating supporting references from 2014 review (MSAC) |
NCSP recommendation |
| Based on NCSP policy |
Consensus-based recommendation |
| Formulated using a consensus process when a systematic review was undertaken and insufficient quality evidence was found on which to base a recommendation |
Practice point recommendation |
| A recommendation on a subject that is outside the scope of the search strategy for the systematic review, based on expert opinion and formulated by a consensus process. |
The Australian Department of Health and the Cancer Council have produced many other resources, such as a short-form of the guidelines, with recommendations only. They have also produced FAQ sheets on many topics related to the new guidelines.
There was a breakfast session on NSCP Renewal at the 2018 RANZCOG Annual Scientific Meeting (ASM) in Adelaide. We identified some common misunderstandings in the management of women with abnormal results and those from specific populations from the scenarios presented to the audience responder session.
Discordant results
It is recognised that all tests requiring human interpretation are subject to a variation in that interpretation. This applies to pathology, as in other fields. There are a few circumstances where pathological review is very worthwhile:
- Adenocarcinoma in situ is difficult to diagnose cytologically, colposcopically and histologically
- A woman with a cytological prediction of high-grade squamous intraepithelial lesion (HSIL) may have a normal colposcopy.
The value of pathological review is acknowledged by a new item number for cytological review. Some fortunate doctors have access to multidisciplinary review meetings. These are to be encouraged. It was disappointing that many of the attendees at the ASM breakfast workshop did not appear to have access to such review meetings.
If cytological review confirms the presence of definite HSIL, then it is highly likely disease is present, but it may be in the vagina, and this should be carefully examined.
Specific populations
Immuno-deficient women
This appeared to be an area of confusion in responses to scenario questions at the breakfast workshop. The new guidelines state that evidence for the safety of lengthening the screening interval in these women is unclear and, in line with other international recommendations, recommend that women be screened at a three-yearly interval. Co-testing is not recommended, as the evidence suggests it offers little additional benefit compared with HPV testing alone.
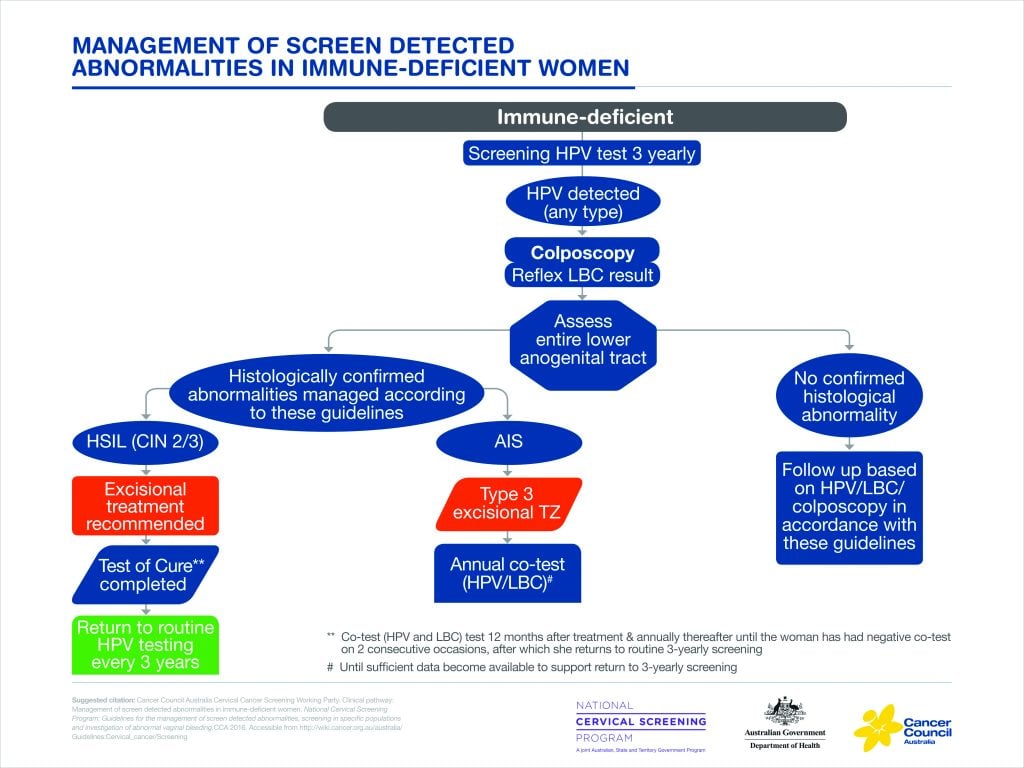
Figure 2. Management of screen-detected abnormalities in immune-deficient women.
Pregnancy
There was good understanding among workshop participants that pregnant women who are due or overdue for screening should be offered it as part of routine antenatal care. The choice of tool for collection of a cervical screening specimen is the broom-type brush. Abnormalities of the CST should be referred for colposcopy as per the guidelines.
The aim of colposcopy in pregnancy is to exclude invasive disease. Cervical biopsy in pregnancy is usually not necessary, provided an experienced colposcopist is confident there is no invasive disease present. Colposcopy in pregnancy can be challenging and should be undertaken by a colposcopist experienced in assessing women during pregnancy. Definitive treatment of suspected high-grade disease, except invasive disease, may be safely deferred until after pregnancy.
Under-screened and never-screened women
It is reported that 80 per cent of cervical cancer in Australia occurs in under-screened or never-screened women. Self-collection of HPV test samples has been suggested as a strategy to overcome the barriers that some women experience. All women who have been sexually active should be screened.
Self-collection is available for women who decline conventional screening and meet the following criteria:
- Have never participated in the NCSP and are 30 years of age or older
- Are overdue for cervical screening by two years or longer and are 30 years of age or over.
Self-collection is not recommended for women who are:
- Symptomatic
- Pregnant
- Diethylstilbestrol (DES)-exposed
- Previously had a hysterectomy with a history of HSIL.
Initially, there was concern that self-collection may not be as sensitive as clinician-collected samples. More recent evidence, such as the Arbyn Meta-analysis,3 suggests that when using only polymerase chain reaction (PCR) techniques for the detection of HPV DNA, the sensitivity of detecting it in a self-collected sample is equivalent to a clinician-collected sample. A self-collection working group has recently been formed to look at all the evidence and to ensure the implementation of this policy reaches those most at risk of cervical cancer, who have previously not complied with conventional screening guidelines. There is much evidence of the acceptability of this method of screening to women.4
Post-hysterectomy
The flow chart for screening after hysterectomy (Figure 3) is very complex in order to incorporate the large numbers of different situations.
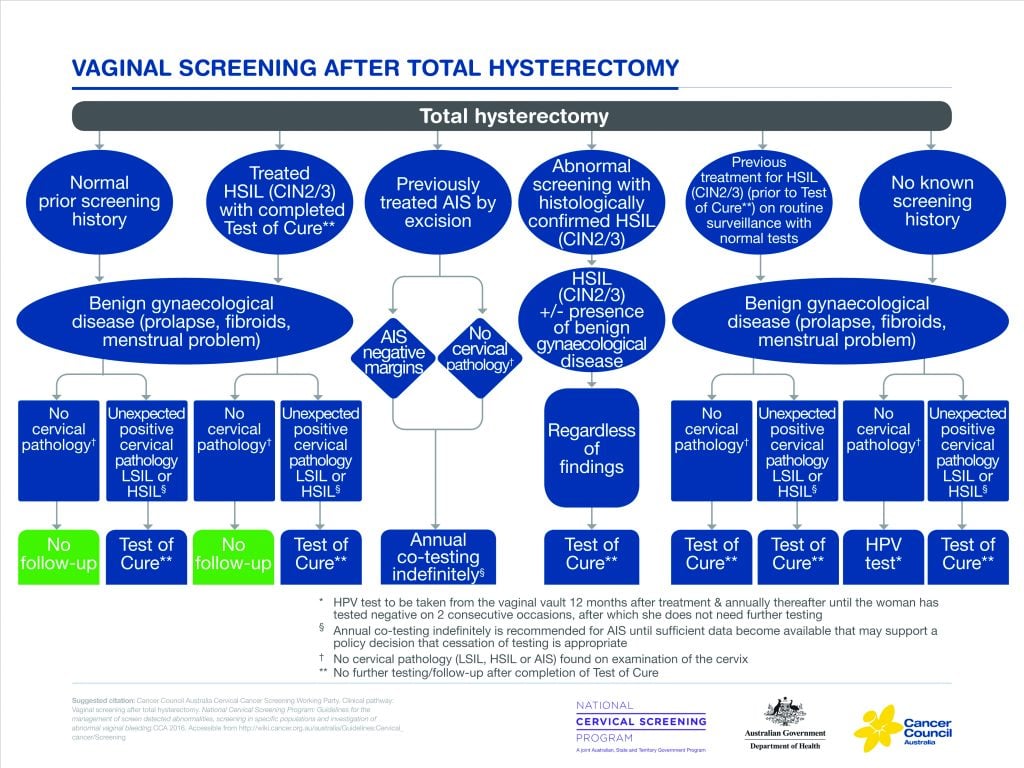
Figure 3. Vaginal screening after total hysterectomy.
If a woman has no history of HSIL and has had negative cytology prior to hysterectomy, with no abnormality in the histology of the cervix in the hysterectomy specimen, then she requires no screening.
For a woman who has a past history of treatment for HSIL and who has completed the test of cure, the advice is the same, provided there is no unexpected abnormality in hysterectomy specimen.
All other women require testing as per the flow chart. Despite the extensive nature of the guidelines, there are always areas that are not entirely clear or new evidence that suggests there should be changes. It must be remembered that these are only guidelines. There may be situations where it seems clinically appropriate to vary the advice of these guidelines. The advantage of the Wiki platform is that the guidelines can be amended as further evidence and technological developments come to light.
Email questions or feedback about the Renewed NCSP to: [email protected].
References
- 2016 Guidelines: http://wiki.cancer.org.au/australia/Guidelines:Cervical_cancer/Screening.
- 2016 Guidelines: http://wiki.cancer.org.au/australia/Guidelines:Cervical_cancer/Screening.
- Arbyn M, Castle PE. Offering self-sampling kits for HPV testing to reach women who do not attend in the regular cervical screening program. Cancer Epidemiol Biomarkers Prev 2015 May;24(5):769-72.
- Gupta S, Palmer C, Bik EM, et al. Self-sampling for HPV testing: increased cervical cancer screening participation and incorporation in international screening programs. Public Health 2018 Apr 9;6:77.






Leave a Reply