Postoperative infection remains the most common complication of gynaecological surgical procedures.1 A surgical site infection (SSI) is any infection that arises within 30 days after an operation in any part of the body where the surgery took place: superficial at the incision site, deep at the incision site or in other organs or spaces opened or manipulated during an operation.2
It is important to consider the likely source of pathogens in each type of surgery. In gynaecological surgery, the source of pathogens can be the endogenous flora of either the patient’s skin or vagina. With laparoscopy or laparotomy, where there is no breach of mucosal surfaces, skin organisms (predominantly Gram-positive organisms such as Staphylococci) are likely.3 4 5 In contrast, with vaginal surgery or hysterectomy, the endogenous flora of the genital tract the likely cause will be polymicrobial, consisting of anaerobes, Gram-negative aerobes and Gram-positive cocci.6 7 8
Prior to any surgery, there is preparation with history, examination and appropriate investigations. Prior to gynaecological surgery, screening for genital tract infection is not required; however, women with symptoms or risk factors should be tested and treated for sexually transmitted infections (chlamydia and gonorrhoea) and bacterial vaginosis. These have been associated with an increased risk of infection, endometritis with chlamydia and gonorrhoea following termination and vaginal cuff infection following hysterectomy with bacterial vaginosis.9 10
Antibiotic prophylaxis should be given to prevent SSI prior to gynaecological surgery or procedures that enter the reproductive tract, ideally 60 minutes prior to skin incision. For procedures such as hysterectomy, antibiotic prophylaxis is clearly indicated, for others such as insertion of IUD or diagnostic laparoscopy, antibiotic prophylaxis is usually not required. For other procedures the evidence is less clear, and recommendations are based on expert agreement until further research evidence becomes available (Table 1).11 12 Readers should note that these recommendations are correct for non-allergic patients, at the time of writing. Surgeons should consider each patient’s individual requirements before prescribing the recommended antibiotic.
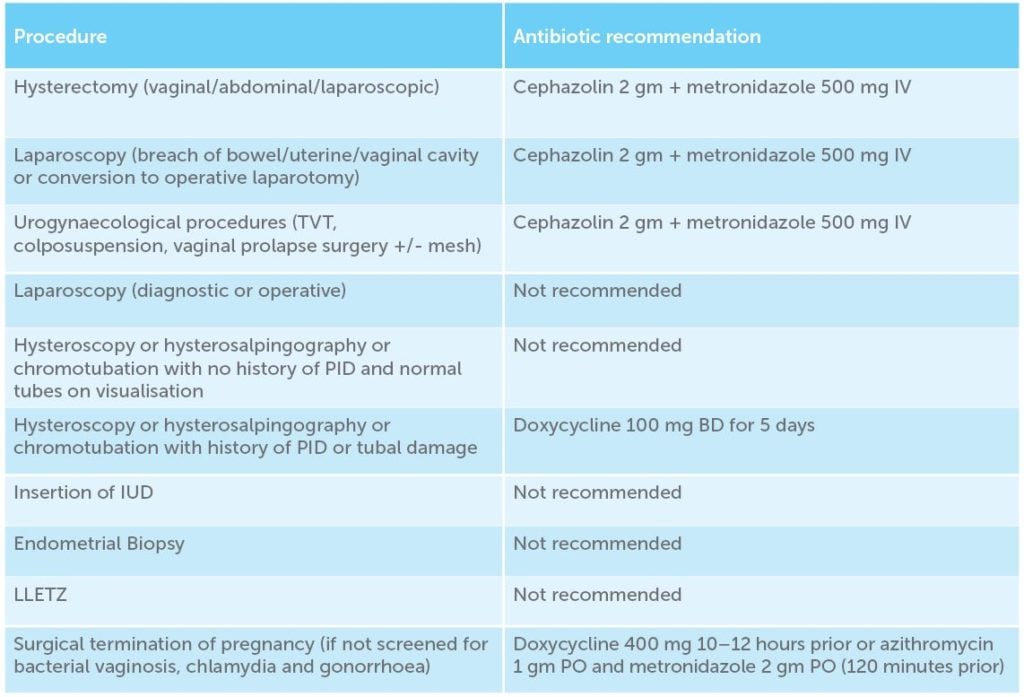
Table 1. Antibiotic recommendation by procedure.
Dosage levels above need to be adjusted for the scenarios including obesity, lengthy procedures and excessive blood loss greater than 1500 mL.13 14
The patient’s risk factors for postoperative infection are predictable and need to be taken into account prior to any surgery, such as smoking status, diabetes, obesity, nutritional status, co-existent infection at a remote body site, vaginal colonisation with micro-organisms and immunodeficiency.15
Other practices recommended to reduce the incidence of infection include:
- Skin preparation: pre-operatively the entire body be washed with soap or an antiseptic agent the night prior. Intra-operatively skin cleansing with chlohexidene-alcohol is superior to povidone-iodine and iodine-alcohol.16 17
- Hair should only be removed at or around the incision site if it will interfere with the operation, preferably with electric clippers. Patients should be advised not to shave the operative site themselves because shaving with a razor increases their risk of infection.18 19 20 21
- Skin preparation: use an alcohol base agent, unless contraindicated.22 23
- Vaginal preparation with either 4% chlorhexidene gluconate or povidone-iodine is acceptable.24
In the operating theatre, measures that have shown to reduce the risk of postoperative infections include: maintaining appropriate aseptic technique, preventing hypothermia, maintaining haemostasis while preserving adequate blood supply, gentle handling of tissue, removal of devitalised tissues, use surgical drains and suture material appropriately, keeping operating time to less than 149 minutes and minimising operative room traffic.25 26
Clinical features and management of gynaecological surgical site infection
Vaginal vault cellulitis
Vaginal cuff infection of the superficial tissues at the vaginal surgical margin after hysterectomy. Symptoms include increasing abdominal pain and purulent yellow vaginal discharge. Physical examination will review hyperaemia and oedema and tenderness out of proportion to what is expected. Treatment is outpatient oral antibiotic therapy with broad spectrum antibiotics, for example, amoxicillin/clavulanate 875/125 mg orally, twice daily.27 28
Superficial skin infection/cellulitis
Wound infection after hysterectomy arises in approximately 1.6 per cent of patients.29 Patients can present with either cellulitis (erythema around the wound) or an incisional abscess (where purulent discharge arises from the incision itself). Diagnosis can be made by clinical examination of the wound, wound swab and wound aspirate (for microscopy, culture and sensitivity). If there is concern for a deeper collection, ultrasound can help exclude an abscess. With an incisional abscess, the wound should be opened and drained and examination of the fascial layer is vital to ensure it remains intact. Management will be antibiotic therapy; whether this is inpatient or outpatient will depend on patient’s clinical status.
Pelvic abscess
Pelvic abscesses arise in less than 1 per cent of patients undergoing obstetric and gynaecological surgery. Patients present with fever, tachycardia, tachypnoea and lower abdominal pain, days to weeks after the hysterectomy. On examination, there will be diffuse pelvic tenderness and sometimes a fluctuant mass may be palpable in the pelvis or vaginal apex. An elevated white cell count with leukocytosis (left shift) and raised inflammatory markers. Imaging will help delineate the abscess with CT (with contrast) having higher sensitivity than pelvic ultrasound. Treatment needs to be prompt and depends on patient’s haemodynamic stability. One possible algorithm to guide treatment is outlined in Figure 1.30 31
Broad spectrum antibiotics need to be commenced according to current local guidelines or after consultation with infectious disease physicians. Intravenous antibiotic therapy should be continued until patient is afebrile for 48 hours, the abscess is reducing in size and the inflammatory markers and the patient are clinically improving. Percutaneous drainage can be performed via ultrasound or CT guidance. Patients who do not respond to appropriate antibiotic treatment require either percutaneous drainage (primary or repeat) or surgery.
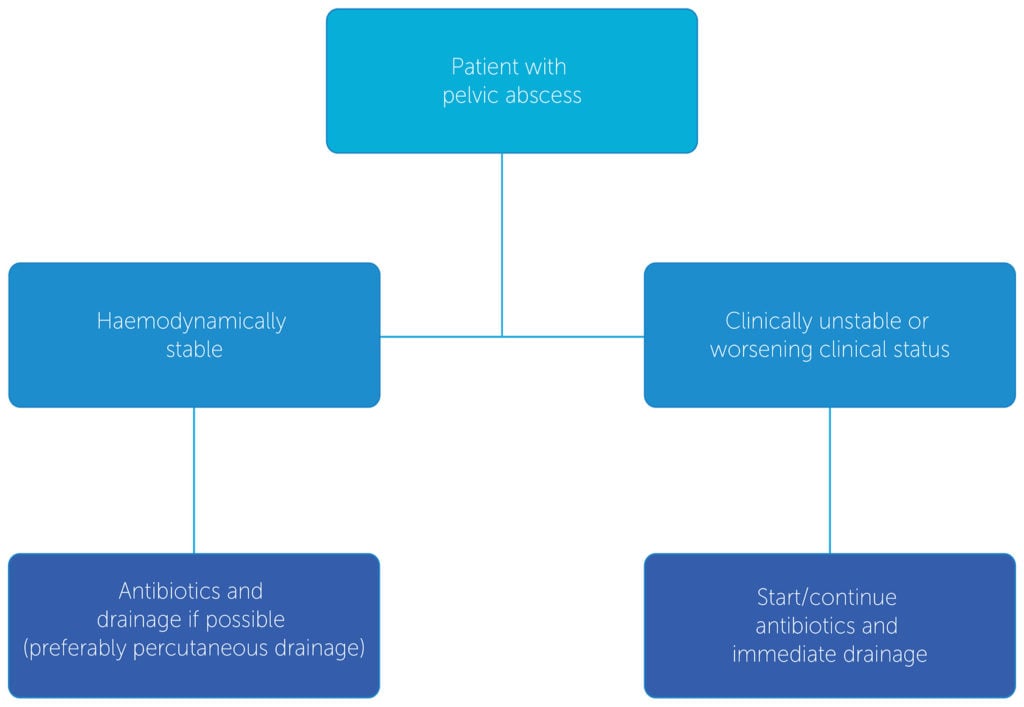
Figure 1. Guiding treatment for pelvic abscess.
Endometritis
Any procedure where there is instrumentation of the uterus has the potential for endometritis. The risk of pelvic infection following surgical termination of pregnancy varies from 0.5–3.5 per cent.32 33 It is, however, relatively uncommon after simple gynaecological procedures such as IUD insertion, hysteroscopy and endometrial sampling.34
The patient with endometritis typically presents with fever, lower abdominal pain and foul-smelling vaginal discharge. On examination, the patient will have a high temperature, rapid pulse and lower abdominal tenderness. On vaginal examination, there may be foul-smelling vaginal discharge and bleeding. Vaginal swabs can be taken and sent for microbiology. Pelvic ultrasound may help differentiate retained products of conception from endometritis.
Conclusion
Postoperative infection is the most common complication of gynaecological surgical procedures. Practitioners should maintain a high level of suspicion of patients presenting with symptoms suggestive of SSI. Individual patient factors may substantially affect the course and treatment of SSI and it is important that prophylaxis, diagnosis and treatment is individualised for each clinical situation.
References
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- Horan TC, Gaynes RP, Martone WJ, et al. CDC definitions of nosocomial surgical site infections, 1992: a modification of CDC definitions of surgical wound infections. Infect Control Hosp Epidemiol. 1992;13(10):606-8.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- Lazenby GB, Soper DE. Prevention, diagnosis, and treatment of gynecologic surgical site infections. Obstet Gynecol Clin North Am. 2010;37(3):379-86.
- Duff P, Park RC. Antiobitic prophylaxis in vaginal hysterectomy: a review. Obstet Gynecol. 1980;55(5):193-202.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- Lazenby GB, Soper DE. Prevention, diagnosis, and treatment of gynecologic surgical site infections. Obstet Gynecol Clin North Am. 2010;37(3):379-86.
- Duff P, Park RC. Antiobitic prophylaxis in vaginal hysterectomy: a review. Obstet Gynecol. 1980;55(5):193-202.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- Miller L, et al. Randomised treatment trial of bacterial vaginosis to prevent post-abortion complication. BJOG. 2004;111(9):982.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- Therapeutic Guidelines Limited. Surgical prophylaxis for gynaecological surgery. In: eTG complete. Melbourne: Therapeutic Guidelines Limited; 2019.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. NICE Guideline (NG25). 2019. Available from: www.nice.org.uk/guidance/ng125.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. NICE Guideline (NG25). 2019. Available from: www.nice.org.uk/guidance/ng125.
- Berrios-Torres SI, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. Healthcare Infection Control Practices Advisory Committee. JAMA Surg. 2017;152:784-91.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. NICE Guideline (NG25). 2019. Available from: www.nice.org.uk/guidance/ng125.
- Berrios-Torres SI, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. Healthcare Infection Control Practices Advisory Committee. JAMA Surg. 2017;152:784-91.
- Tanner J , et al. Preoperative hair removal to reduce surgical site infection. Cochrane Database Syst Rev. 2011;11: CD004122. (Meta-Analysis).
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- Berrios-Torres SI, et al. Centers for disease control and prevention guideline for the prevention of surgical site infection, 2017. Healthcare Infection Control Practices Advisory Committee. JAMA Surg. 2017;152:784-91.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- ACOG Practice Bulletin No. 195: Prevention of Infection After Gynecologic Procedures.. Obstet Gynecol. 2018;131(6):e172-89.
- National Institute for Health and Care Excellence. Surgical site infections: prevention and treatment. NICE Guideline (NG25). 2019. Available from: www.nice.org.uk/guidance/ng125.
- Lazenby GB, Soper DE. Prevention, diagnosis and treatment of gynaecologic surgical site infections. Obstetrics and Gynecology Clinics of North America. 2010; 77(3):379-386
- Lachiewicz MP, Moulton LJ, Jaiyeoba O. Pelvic surgical site infections in gynecologic surgery. Infect Dis Obstet Gynecol. 2015;2015:614950.
- Lake AG, McPencow AM, Dick-Biascoechea MA, et al. Surgical site infection after hysterectomy. Am J Obstet Gynecol. 2013;209(5):490.e1-9.
- Lachiewicz MP, Moulton LJ, Jaiyeoba O. Pelvic surgical site infections in gynecologic surgery. Infect Dis Obstet Gynecol. 2015;2015:614950.
- Jaiyeoba O. Postoperative infections in obstetrics and gynaecology. Clin Obstet Gynecol. 2012;55:904.
- Al-Ma’ani W, et al. Expectant versus surgical management of first-trimester miscarriage: a randomized controlled study. Arch Gynecol Obstet. 2014;289:1011-5.
- Russo J, et al. Controversies in Family Planning: Postabortal pelvic inflammatory disease. Contraception. 2013;87(4):497-503.
- Propst AM, et al. Complications of hysteroscopic surgery: predicting patients at risk. Obstet Gynecol. 2000;96(4):517.






Leave a Reply