The ovaries are a key organ of female reproduction, necessary for the storage and release of the oocytes – the egg cells that in adulthood may be fertilised by sperm to develop into an embryo. The ovaries also produce and secrete female sex hormones that are essential for the development of secondary sex characteristics and for regulation of the menstrual cycle.
Ovarian development
The ovary begins to develop at approximately 6–7 weeks of gestation. By 20 weeks, each ovary contains a peak of around 5 million oocytes.1 This oocyte population starts to decline precipitously from this time, with around 1–2 million oocytes at birth, each stored inside a single cell layer envelope, known as a follicle, inside the ovary. Immediately after birth, this population of oocytes continues to decline so that by the onset of puberty a girl retains around 180,000.2 While the diminution of this initial population is necessary to eliminate those with DNA damage or dysfunctional mitochondria3 and to allow the surviving oocytes to be supported in a healthy condition, several genetic and environmental factors can influence how many oocytes survive to this stage. Genetic perturbations in such genes as Nobox, Figla and Taf4b, have been implicated in primary ovarian insufficiency.4 Likewise, maternal smoking reduces the number of oocytes in the fetal ovary5 and endocrine disruptors, including synthetic oestrogens (such as bisphenol A [BPA], found in plastics), and the phytoestrogen genistein (a key ingredient in soybeans), can impair follicle formation and contribute to abnormal oocyte retention.6
The immune system also plays an important role in the multifaceted process of the establishment of the initial follicle pool and subsequent follicle development. Interactions between cytokines, growth and transcription factors mediate the assembly and development of the primordial follicle pool.7 8 In our preclinical work, we have shown that an early life immune challenge during the final stages of primordial follicle pool formation results in upregulation of intraovarian cellular pathways associated with immune signalling, leading to increased follicle apoptosis.9 10 Since the number of primordial follicles is finite, a smaller primordial follicle reserve is typically associated with a shorter reproductive lifespan and reduced fertility.11 Our current research utilises CRISPR/Cas9 targeted genome editing in animal models to investigate the role of the major immune cells in ovarian development and function.
In the adult, ovaries are egg-shaped structures approximately 4 cm long. They lie on either side of the uterus, against the pelvic wall, in a region known as the ovarian fossa. They are attached to the uterus by the ovarian ligament and to the fallopian tube by the infundibulopelvic ligament. The ovary is not directly attached to the fallopian tube, but releases the oocyte into peritoneal fluid produced by fallopian tube fimbriae, with the oocyte picked up by cilia on the fimbriae.12 The oocyte then travels down the fallopian tube where it can be fertilised before moving to the uterus to implant in the uterine wall (Figure 1).
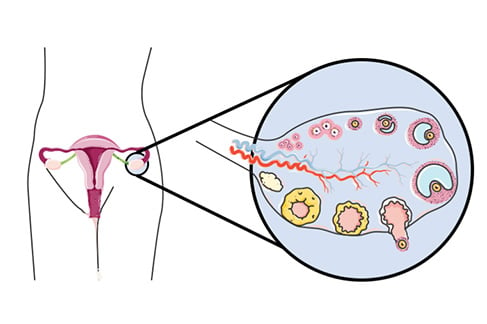
Figure 1. Position of the ovary in the body (circled) with a magnification of the ovary showing all stages of follicular maturation. Clockwise from smallest: primordial to attrition of the corpus luteum.
Follicle maturation and oocyte release
Which oocytes from the initial pool end up being fertilised depends upon which follicles make it through to the appropriate maturation stage at the correct phase of the menstrual cycle. The initial recruitment of the primordial follicles into the developing pool is regulated in part by anti-Müllerian hormone (AMH), which inhibits activation. This, and each additional stage of follicle development, requires the contribution of a host of different hormones and growth factors and the expression of different receptors on the cells of the follicle and on the oocyte itself.13 As the follicle matures, the single cell layer enclosing the oocyte divides rapidly and the follicle becomes progressively larger. The maturation of each ovarian follicle from the primordial to the pre-ovulatory stage takes several months and follicles undergo folliculogenesis at all stages of the menstrual cycle, so that follicles of all developmental stages are present at all times.14 There is relatively high attrition during this process, with only a small portion of follicles that commence maturation progressing to ovulation. Midway through each menstrual cycle, gonadotrophin-releasing hormone (GnRH) is released from GnRH neurons in the hypothalamus, stimulating the pulsatile release of follicle stimulating hormone (FSH) and luteinising hormone (LH). These hormones circulate in the bloodstream and act on the ovary to stimulate ovulation.15 In response to high pulsatile levels of LH, the ovary releases an oocyte from the most mature of the follicles into the reproductive tract, where it can be fertilised. The empty follicle becomes the corpus luteum that releases progesterone to stimulate uterine development in preparation for a potential pregnancy. Due to the high attrition of ovarian follicles through the maturation process a woman only ovulates around 400–500 oocytes, giving her a mean reproductive lifespan of approximately 35 years.16
The ovary and sex hormones
In addition to being the storehouse for oocytes, the ovary also produces the major female sex hormones oestrogen, particularly 17β-estradiol, and progesterone. These play a role in follicle growth, but are also important for secondary sex characteristics such as breast development, hip and thigh fat deposition, as well as for regulating the menstrual cycle. 17β-estradiol is produced by granulosa cells of the ovary, increasing in circulation from the start of the menstrual cycle until the pre-ovulatory phase where it acts at the hypothalamus to stimulate GnRH-mediated pulse generation, culminating in LH release, which in turn triggers ovulation. Progesterone is produced by the corpus luteum and so is low in the preovulatory phase of the menstrual cycle, increasing after ovulation where it supports the pregnancy should fertilisation occur.17 Oestrogen and progesterone are not only important in regulating menstruation and preparing the uterus for pregnancy, both have a host of additional functions.18 For example, oestrogen plays a role in cognitive function in women. When oestrogen levels are decreasing and progesterone rising, in the luteal phase of the menstrual cycle, women preferentially use a spatial strategy to solve a navigation task. At early stages of the menstrual cycle they (with the same success levels) preferentially use a response strategy in the same task19 (Figure 2). These adjustments may be achieved by oestrogen-mediated differences in the density of synapses, which, at least in animal models, can differ by up to 30% across the ovarian cycle.20
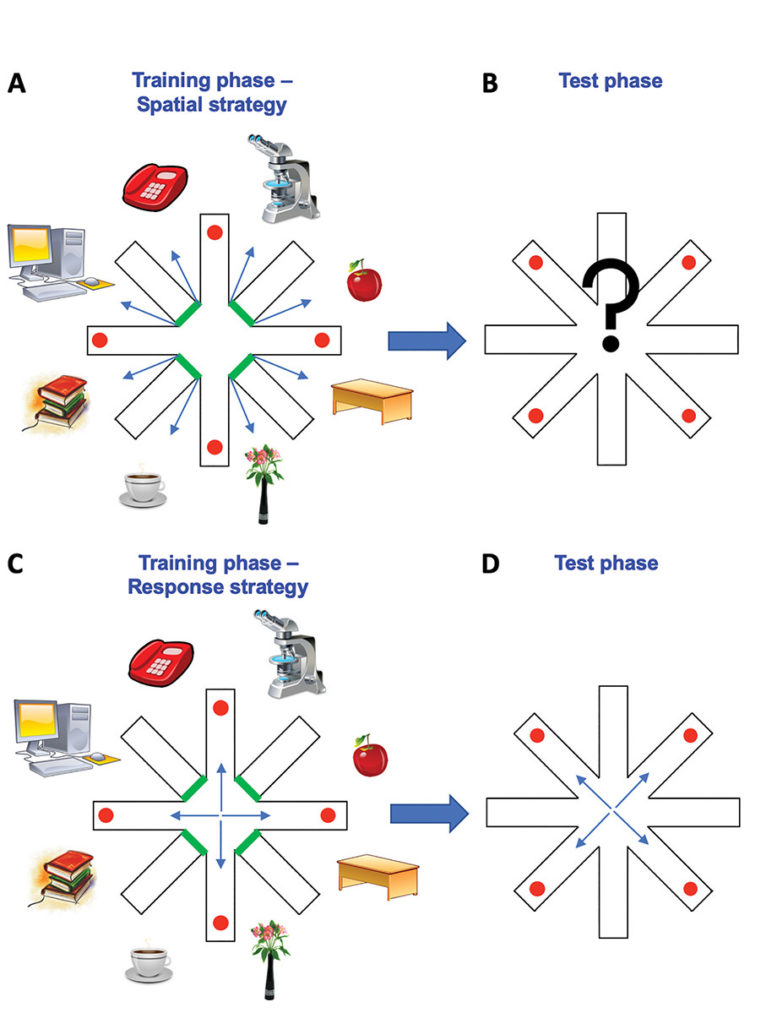
Figure 2. Illustration of decision-making strategies in cognitive tasks during the different stages of the menstrual cycle. During the training phase of the task, participants are introduced to a radial arm maze, consisting of eight arms with objects at the end of four accessible arms. Participants are required to remember which arms they visited in the training phase in order to avoid these arms and collect objects from different arms in the test phase. In the luteal phase of the cycle, high levels of progesterone boost a reliance on landmarks in the environment leading to implementation of a spatial strategy (A). This strategy relies on associations between landmarks, creating a cognitive map of the environment. If these landmarks are removed in the test phase (B), this will result in an increase in errors. In the pre-ovulatory and ovulatory phases of the cycle, rising and high oestrogen levels, respectively, drive a response strategy that relies on stimulus-response specific associations. Therefore, a removal of landmarks in the test phase (D), results in minimal errors.
Ovarian dysfunction
Most women naturally undergo reproductive senescence at around the age of 50. This occurs due to a combination of the depletion of the follicle pool and a decline in the production of sex hormones. As a woman ages, the remaining oocytes can also accrue double-strand DNA damage due to a decline in the expression of key genes normally involved in DNA repair. Therefore, fertility becomes increasingly less likely as we age. Genetic and environmental factors interact to contribute to the rate of ovarian ageing. Latest genome-wide association studies identify at least 44 gene loci that influence when a woman undergoes menopause. Added to this, poor diet, environmental toxins, stress and illness can all lead to ovarian inflammation, oxidative stress and premature follicular atresia.21 An integration between the environment and genetic background may be achieved via non-coding RNAs, explaining why some women are more susceptible to these influences than others.
A further major cause of ovarian dysfunction is polycystic ovary syndrome (PCOS). This condition affects 5–10% of women of reproductive age and is more common in obese women. In this condition, the follicles mature to the antral stage but fail to progress further and the oocyte is not ovulated. These follicles appear as eponymous cysts on the ovary under ultrasound examination. The condition is associated with a number of complications and is thought to be caused by an imbalance in ovarian hormones, with contribution from other hormones, such as insulin. Lifestyle factors, including prenatal hyperandrogenism and diet at any life stage, can exacerbate PCOS.22
Infertility also occurs in relatively young healthy women for whom causes are difficult to define. Stress may be one of these causes. Stress is fairly ubiquitous in all our lives, making it difficult to ascertain the full impact on ovarian health; and, certainly, women with significant life stress do usually have functional ovaries and normal fertility. However, evidence suggests that the interplay between stress and other lifestyle and physiological factors may contribute to ovarian dysfunction. As one example, stress in conjunction with a dysregulation in the signalling of the satiety hormone, ghrelin, may lead to impaired ability of the ovary to synthesize sex hormones and to premature follicular development and attrition.23 Our preclinical work has shown the ability of the ghrelin receptor antagonist to attenuate stress-induced depletion of primordial follicles.24 This work suggests there may be a link between eating, stress and ovarian dysfunction. While adequate nutrition and energy balance are essential for reproductive function at all stages of life, it is particularly important during early development, with both insufficient and excess dietary intake altering reproductive development and ovarian function, and impeding adult fertility.25 Our group and others have shown that early life under- or overnutrition in rodents alters the timing of puberty,26 impairs ovulatory capacity and accelerates the depletion of the primordial follicle reserve.27 These studies indicate that a poor early life nutritional environment can cause lasting and deleterious effects on the development of reproductive circuitry, including the ovary, increasing the potential to experience lifelong reproductive dysfunction.
Future research
The aetiologies of PCOS and premature ovarian insufficiency are still not well understood and our capacity to remedy these is currently poor. We are also in want of strategies for preventing and reversing the effects of ageing on the oocyte. New research into the capacity for oogonial stem cells to generate healthy eggs and offspring is an exciting direction for the field in this regard.28 Perhaps most important is the need to identify markers for an early diagnosis of ovarian dysfunction, not only for therapy management, but so that women can make informed reproductive choices.
References
- Findlay JK, Hutt KJ, Hickey M, Anderson RA. How Is the Number of Primordial Follicles in the Ovarian Reserve Established? Biol Reprod. 2015;93(5):111.
- Findlay JK, Hutt KJ, Hickey M, Anderson RA. How Is the Number of Primordial Follicles in the Ovarian Reserve Established? Biol Reprod. 2015;93(5):111.
- Krakauer DC, Mira A. Mitochondria and germ-cell death. Nature. 1999;400(6740):125-6.
- Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142(15):2554-63.
- Findlay JK, Hutt KJ, Hickey M, Anderson RA. How Is the Number of Primordial Follicles in the Ovarian Reserve Established? Biol Reprod. 2015;93(5):111.
- Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142(15):2554-63.
- Feeney A, Nilsson E, Skinner MK. Cytokine (IL16) and tyrphostin actions on ovarian primordial follicle development. Reproduction. 2014;148(3):321-31.
- Pepling ME. Follicular assembly: mechanisms of action. Reproduction. 2012;143(2):139-49.
- Sominsky L, Sobinoff AP, Jobling MS. Immune regulation of ovarian development: programming by neonatal immune challenge. Frontiers in Neuroscience. 2013;7:100.
- Fuller EA, Sominsky L, Sutherland JM, et al. Neonatal immune activation depletes the ovarian follicle reserve and alters ovarian acute inflammatory mediators in neonatal rats. Biol Reprod. 2017; 97(5):719-30.
- Findlay JK, Hutt KJ, Hickey M, Anderson RA. How Is the Number of Primordial Follicles in the Ovarian Reserve Established? Biol Reprod. 2015;93(5):111.
- Lyons RA, Saridogan E, Djahanbakhch O. The reproductive significance of human Fallopian tube cilia. Hum Reprod Update. 2006;12(4):363-72.
- Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci. 2003;78(3-4):135-63.
- Fortune JE. The early stages of follicular development: activation of primordial follicles and growth of preantral follicles. Anim Reprod Sci. 2003;78(3-4):135-63.
- Sominsky L, Hodgson DM, McLaughlin EA, et al. Linking Stress and Infertility: A Novel Role for Ghrelin. Endocrine Reviews. 2017;38(5):432-67.
- Grive KJ, Freiman RN. The developmental origins of the mammalian ovarian reserve. Development. 2015;142(15):2554-63.
- Evans J, Salamonsen LA, Winship A, et al. Fertile ground: human endometrial programming and lessons in health and disease. Nat Rev Endocrinol. 2016;12(11):654-67.
- Barrientos RM, Brunton PJ, Lenz KM, et al. Neuroimmunology of the female brain across the lifespan: Plasticity to psychopathology. Brain Behav Immun. 2019;79:39-55.
- Hussain D, Hanafi S, Konishi K, et al. Modulation of spatial and response strategies by phase of the menstrual cycle in women tested in a virtual navigation task. Psychoneuroendocrinology. 2016;70:108-17.
- Woolley CS, Gould E, Frankfurt M, McEwen BS. Naturally occurring fluctuation in dendritic spine density on adult hippocampal pyramidal neurons. J Neurosci. 1990;10(12):4035-9.
- Cuomo D, Ambrosino C. Non-coding RNAs as integrators of the effects of age, genes, and environment on ovarian aging. Cell Death Dis. 2019;10(2):88.
- Filippou P, Homburg R. Is fetal hyperexposure to androgens a cause of PCOS? Hum Reprod Update. 2017;23(4):421-32.
- Sominsky L, Hodgson DM, McLaughlin EA, et al. Linking Stress and Infertility: A Novel Role for Ghrelin. Endocrine Reviews. 2017;38(5):432-67.
- Di Natale MR, Soch A, Ziko I, et al. Chronic predator stress in female mice reduces primordial follicle numbers: implications for the role of ghrelin. Journal of Endocrinology. 2019;241(3):201-19.
- Sloboda DM, Hickey M, Hart R. Reproduction in females: the role of the early life environment. Hum Reprod Update. 2011;17(2):210-27.
- Caron E, Ciofi P, Prevot V, Bouret SG. Alteration in neonatal nutrition causes perturbations in hypothalamic neural circuits controlling reproductive function. J Neurosci. 2012;32(33):11486-94.
- Sominsky L, Ziko I, Soch A, et al. Neonatal overfeeding induces early decline of the ovarian reserve: Implications for the role of leptin. Molecular and Cellular Endocrinology. 2016;431:24-35.
- Martin JJ, Woods DC, Tilly JL. Implications and Current Limitations of Oogenesis from Female Germline or Oogonial Stem Cells in Adult Mammalian Ovaries. Cells. 2019;8(2). pii: E93.







Leave a Reply