A woman with a previous caesarean section and anterior placenta praevia is referred with a suspicion of placenta accrete. What components are required for planning someone’s birth with placenta accrete spectrum (PAS) disorder?
Placenta accreta spectrum (PAS) disorders encompass both adherent (placenta accreta) and invasive placental pathology (increta and percreta). PAS abnormalities have increased in tandem with the rise in caesarean delivery rates with a near 10-fold increase over the last four decades.1 The majority of cases of PAS result in preterm birth, one-in-two require hysterectomy, involve a major obstetric haemorrhage and/or transfusion, and one-in-three need ICU admission. Thus, PAS is now recognised as a major cause of obstetric morbidity requiring multidisciplinary, coordinated care and delivery planning.
Prenatal diagnosis
Antenatal suspicion of the possibility of an abnormally adherent placenta is crucial as maternal and perinatal outcomes are optimised when planned birth occurs in units where there is expert surgical and anaesthetic expertise, intensive care and transfusion facilities available.2 Despite the variability of PAS abnormalities, obstetric ultrasonography performed by skilled operators is highly accurate in making the diagnosis. Hence, if there is concern about the appearance of the placenta, referral to a specialist imaging unit is recommended.3
The International Society for Ultrasound in Obstetrics and Gynaecology have published guidelines that detail ultrasound features necessary for diagnosis.4These include multiple vascular lacunae within the placenta, loss of the normal hypoechoic placental/myometrial interface (clear zone), abnormalities of the uterine serosa/bladder interface, the presence of myometrial thinning or placental bulging and increased vascularity of colour Doppler.5 Although the mainstay of diagnosis is ultrasound, Magnetic Resonance Imaging (MRI) is a complementary imaging modality and may have a role to play in cases of posterior PAS disorders and placenta percreta.6
Management after prenatal diagnosis
Access to skilled multidisciplinary team (MDT) usually requires referral to, and birth in, a tertiary facility. Given the significant risk of high volume blood loss and preterm birth, there is a need for adult and neonatal intensive care facilities, rapid transfusion blood bank access and the availability to a multidisciplinary surgical team with PAS disorder experience comprising obstetricians, neonatologists, anaesthetists, and expert pelvic surgeons.7 Ideally, once an at-risk patient is identified, MDT review and counselling should be undertaken as soon as possible, particularly if support may be required from colorectal and vascular surgeons and/or interventional radiologists.
At our institution, the diagnosis of PAS immediately triggers a standardised approach involving referrals to the gynae-oncology and anaesthetic teams. Additionally, an ICU bed is booked, extended theatre time with cell saver access is arranged and the blood bank is notified to ensure that patient-specific blood is available. The involvement of gynae-oncologists is driven by local expertise and evidenced by retrospective case-series suggesting the presence of a gynae-oncologist at the beginning of a PAS case is associated with reduced blood loss.8
Box 1. Considerations in the management of PAS disorders.
Pre-operative
- Prenatal consultations by relevant speciality/subspeciality (anaesthetics, gynae-oncology)
- Plan specific timing of delivery
- Plan location of delivery and associated logistical support (ICU, cell saver, blood bank)
- Maximisation of pre-operative haemoglobin
- Consideration for temporary relocation of patient and family closer to surgical centre or provide information regarding plan in the presence of bleeding
- Consider maternal steroids
- Blood cross matched pre-operatively
Intraoperative
- Consideration of peri-operative ultrasound to help plan surgical approach
- MDT consultation within OT (team huddle, operative set up, use of appropriate equipment, haemostatic agents, and discussion of remedial measures in the event of heavy bleeding for uterine sparing surgery)
- Confirm the presence of cell saver and blood bank/blood products
Postoperative
- Debrief and careful post-surgical care
- Prolonged DVT prophylaxis after surgery
- Follow up planning if conservative or uterine sparing techniques used
When should delivery be planned?
There is a wide variation in timing of birth for these women ranging from 34–38 weeks. 9 10 11 12 In all cases, planned birth is essential as this approach has been shown to have lower maternal and perinatal complications compared to emergency care.13 The timing of birth needs to be balanced against the possibility of an acute, out-of-hours admission and its attendant issues. As stated, planned preterm birth reduces the likelihood of an emergency presentation; however, this must be weighed against the increased risks of iatrogenic prematurity and its implications for the neonate.
The risks of unplanned preterm birth are higher in women with risk factors such as previous preterm birth, prior antepartum-partum haemorrhage, and in the presence of prelabour rupture of membranes. 14 Thus, planned delivery between 34+0 and 36+0 weeks may be reasonable in women with significant risk factors for preterm birth. In those without risk factors, planned birth between 36–37 weeks gestation is feasible. 15
Adjunct pre-operative planning
PAS disorders are associated with heavy bleeding and optimisation of maternal medical conditions, especially anaemia, prior to delivery is indicated.
Although there is no evidence for antenatal hospitalisation of asymptomatic patients,16 tailoring care to individuals with specific requirements such as geographical isolation will sometimes mean relocation closer to the time of delivery, particularly where PAS co-exists with placenta praevia. Along with surgical planning, early involvement of social workers may assist in the organisation of accommodation and support structures.
In view of the likely need for preterm operative delivery, steroids for fetal maturity are considered as close as possible to the planned date of surgery.
Is a caesarean hysterectomy always required?
Generally, attempts to remove even a mildly adherent placenta increases the risk of haemorrhage. Therefore, options of management of PAS fall into one of three main categories: conservative management, uterine-sparing surgical techniques and caesarean hysterectomy each with their advantages and attendant risks (see Table 1).
Retrospective studies of uterine-conserving techniques demonstrate relatively high rates of infectious and bleeding morbidity during prolonged monitoring and follow up. Expectant management alone has yielded variable success rates defined by uterine conservation of 60–85% with about 6% chance of significant maternal morbidity.17
| Conservative management (placenta in-situ) | Uterine-sparing surgical techniques | Caesarean hysterectomy | |
| Goal | Retain fertility; reduce surgical morbidity |
Reduce surgical morbidity; retain fertility | Definitive therapy |
| Requirements | 1–12 months follow up (mean ~6 months) | 50% or less of anterior myometrium involved, no lateral or cervical invasion | Expert surgical skillset |
| Risks |
|
|
|
One approach in carefully selected patients is partial resection of the affected placental bed. These include the one-step resective-conservative surgery which consists of resecting the invasive accreta area and placenta en-bloc followed by immediate uterine reconstruction.18 Another novel uterine-sparing procedure for PAS disorders is the Triple P-procedure: Perioperative placental localisation, delivery of fetus above upper border of placenta, Pelvic devascularisation and Placental non-separation with myometrial excision.19Reduced rates of maternal morbidity and hysterectomy have been shown in small series comparing triple P to other uterine-preserving approaches and caesarean hysterectomy.20 21 In contrast to cases of caesarean hysterectomy, most uterine-conserving surgery series have involved obstetricians with PAS expertise as primary surgeons with support from gynae-oncological colleagues. Local resection therefore appears reasonably successful and feasible and could be considered in carefully selected cases. 22 23
Whilst some novel techniques are promising, caesarean hysterectomy with placenta left in-situ remains the generally accepted approach in guidelines and is done by 50–70% of clinicians in global surveys.24 This includes when PAS is suspected during routine caesarean section.25
What are the key intraoperative considerations at a caesarean hysterectomy?
Intraoperative planning begins with anaesthetic setup and is usually either a combined spinal-epidural or general anaesthetic. Historically, most patients with PAS disorders were managed with general anaesthesia. However, more recent experience supports the safety of regional anaesthesia with several studies indicating lower or no difference in haemorrhage-related morbidity, improved early neonatal respiratory outcomes, the capacity for the woman to be awake for birth and the capacity to convert to general anaesthetic if required.26
A recent meta-analysis demonstrated that administration of tranexamic acid before CS delivery reduced intra and postoperative blood loss with no increase in thromboembolic events.27 Although these trials did not specifically address PAS disorders, discussion about the pre-operative use of TXA in the surgical management of PAS disorders should be considered. Oxytocin is not provided due to the possibility that partial separation of the placenta may lead to increased blood loss. However, in the event of heavy bleeding its use along with other uterotonic agents and TXA is recommended.
Women are ideally placed in a dorsal lithotomy position or legs straight but parted position to allow access to the vagina and easier assessment of vaginal blood loss.28 Peri-operative ultrasound assessment of fetal lie and placental location is undertaken, prior to commencement of the incision or intraoperatively using a sterile ultrasound probe, to plan the hysterotomy incision distant from the placental bed.
At our institution, a midline skin incision is routinely employed for our PAS cases, although studies have employed both midline or wide transverse incisions depending on many considerations including the location of the placenta, planned hysterotomy site, maternal habitus, likelihood of operative complications and institutional protocols.29 After peritoneal entry, the hysterectomy is commenced prior to hysterotomy: the bladder is mobilised as low as possible, round ligaments are ligated bilaterally and pelvic sidewalls opened. Where possible, ureters are identified and lateralised. If frank invasion of the bladder is suspected, ureteric catheters are placed to assist later dissection steps and, in some cases, deliberate cystotomy is performed.30
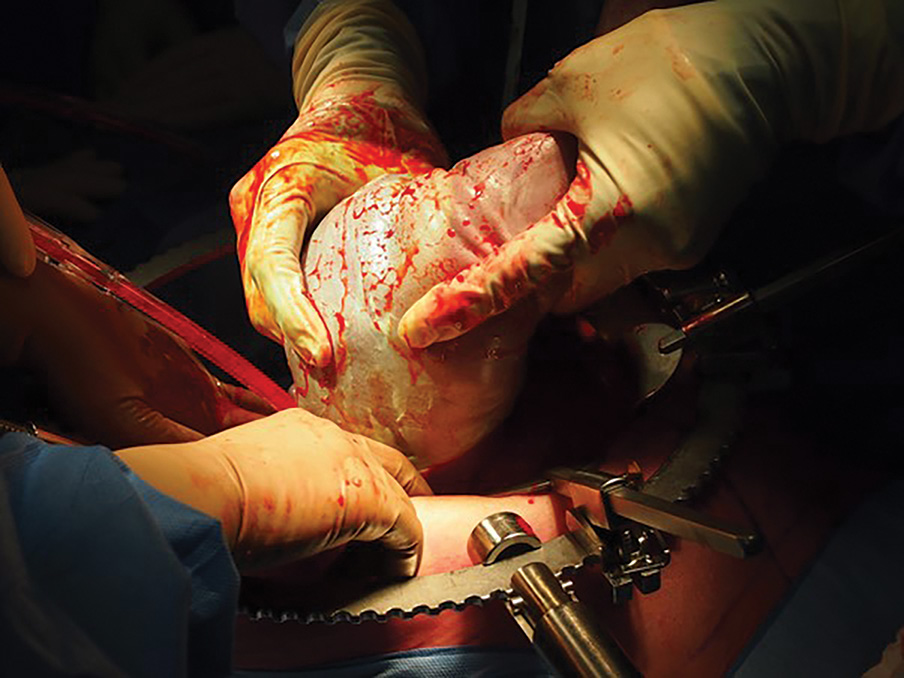
Figure 1. Breech extraction of a fetus during an elective caesarean hysterectomy. A Brookwalter self-retaining retractor system is used here to provide improved operative access.
The fetus is delivered by either a transverse or vertical uterine incision usually in the fundus above the placental implantation site, followed by clamping of the cord close to the placenta and uterine closure (Figure 1). Hysterectomy proceeds until the level of the cardinal ligaments when a narrow Deaver retractor is placed in the vagina to identify the anterior vaginal fornix. The anterior vagina is then opened, and the hysterectomy finished in a retrograde fashion (Figure 2).
Continuous intraoperative appraisal of blood loss and patient volume status is crucial, along with the use of cell-saver and utilisation of massive transfusion protocols as required. The role of pre-operative pelvic artery balloon catheter placement remains controversial and is not utilised at our institution. Access to arterial embolisation peri-operatively, whilst not often required in cases of caesarean hysterectomy, may be useful for conservative or uterine-sparing surgical approaches.
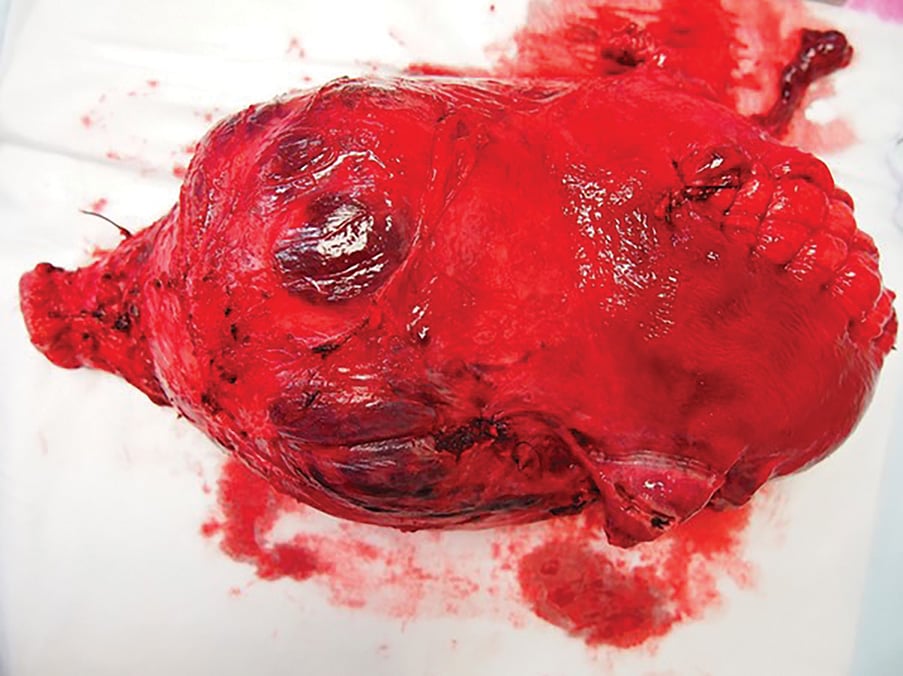
Figure 2. Uterus following caesarean hysterectomy for PAS. Of note: the midline fundal hysterotomy for delivery of the fetus and the extensive area of increta extending from the right anterolateral wall towards the left lateral wall.
Post-operative care includes analgesia, vigilant post-operative monitoring, thromboprophylaxis, debriefing and follow-up of uterine preserving cases.
In summary, the high-risk nature of PAS deliveries requires a systematic approach to management. Pre-operative diagnosis of the type and extent of PAS is crucial, after which, a thorough discussion of the options of management should occur incorporating both the experience of the clinical team and the patient’s wishes including her desire for future fertility. Careful pre-operative planning and care in a centre with an experienced MDT, immediate availability of blood products, access to adult and neonatal intensive care are all essential to optimise outcomes for both mother and baby in PAS disorders.
We would like to thank Dr Nimithri Cabraal and Prof Sailesh Kumar for their review of this article prior to submission.
References
- Solheim K, Esakoff T, Little S, et al. The effect of caesarean delivery rates on the future incidence of placenta previa, placenta accreta, and maternal mortality. Journal of Maternal-Fetal & Neonatal Medicine. 2011;24:1341-6.
- Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstetrics and Gynecology. 2018;132(6)
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Jauniaux E, Bhide A, Kennedy A, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. International Journal of Gynecology and Obstetrics. 2018;140(3).
- Jauniaux E, Bhide A, Kennedy A, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Prenatal diagnosis and screening. International Journal of Gynecology and Obstetrics. 2018;140(3).
- Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstetrics and Gynecology. 2018;132(6)
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Brennan DJ, Schulze B, Chetty N, et al. Surgical management of abnormally invasive placenta: A retrospective cohort study demonstrating the benefits of a standardized operative approach. Acta Obstetricia et Gynecologica Scandinavica. 2015;94(12).
- Obstetric Care Consensus No. 7: Placenta Accreta Spectrum. Obstetrics and Gynecology. 2018;132(6).
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Allen L, Jauniaux E, Hobson S, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Nonconservative surgical management. International Journal of Gynecology and Obstetrics. 2018;140(3).
- Jauniaux ERM, Alfirevic Z, Bhide AG, et al. Placenta Praevia and Placenta Accreta: Diagnosis and Management: Green-top Guideline No. 27a. BJOG. 2019;126(1).
- Jauniaux ERM, Alfirevic Z, Bhide AG, et al. Placenta Praevia and Placenta Accreta: Diagnosis and Management: Green-top Guideline No. 27a. BJOG. 2019;126(1).
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Palacios-Jaraquemada JM, Diagnosis and management of placenta accreta. Best Pract Res Clin Obstet Gynaecol. 2008;22:1133-48.
- Chandraharan E, Rao S, Belli AM, Arulkumaran S. The Triple-P procedure as a conservative surgical alternative to peripartum hysterectomy for placenta percreta. International Journal of Gynecology and Obstetrics. 2012;117(2).
- Teixidor Viñas M, Belli AM, Arulkumaran S, Chandraharan E. Prevention of postpartum hemorrhage and hysterectomy in patients with morbidly adherent placenta: A cohort study comparing outcomes before and after introduction of the Triple-P procedure. Ultrasound in Obstetrics and Gynecology. 2015;46(3).
- Pinas-Carrillo A, Bhide A, Moore J et al., Outcomes of the first 50 patients with abnormally invasive palcenta managed using the “triple P Procedure” conservative surgical approach. Internal Journal of Gynaecology & Obstetrics. 2020; 148:65-71
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Sentilhes L, Kayem G, Chandraharan E, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Conservative management. International Journal of Gynecology and Obstetrics. 2018;140(3).
- Allen L, Jauniaux E, Hobson S, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Nonconservative surgical management. International Journal of Gynecology and Obstetrics. 2018;140(3).
- Allen L, Jauniaux E, Hobson S, et al. FIGO consensus guidelines on placenta accreta spectrum disorders: Nonconservative surgical management. International Journal of Gynecology and Obstetrics. 2018;140(3).
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Simonazzi G, Bisulli M, Saccone G et al., Tranexamic acid for preventing postpartum blood loss after caesarean delivery: A systematic review and meta-analyses of randomized controlled trials. Acta Obstet Gynaecol Scand. 2016;95:28-37
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Collins SL, Alemdar B, van Beekhuizen HJ, et al. Evidence-based guidelines for the management of abnormally invasive placenta: recommendations from the International Society for Abnormally Invasive Placenta. American Journal of Obstetrics and Gynecology. 2019;220(6).
- Brennan DJ, Schulze B, Chetty N, et al. Surgical management of abnormally invasive placenta: A retrospective cohort study demonstrating the benefits of a standardized operative approach. Acta Obstetricia et Gynecologica Scandinavica. 2015;94(12).






Leave a Reply