Most miscarriages result from chromosomal variations in the embryo. The increasing difficulty that older women have in conceiving and their higher risk of miscarriage is also thought to result from the higher rate of non-disjunction in the ageing oocytes leading to aneuploid embryos (Figure 1).1 It therefore seems intuitive that testing the chromosome number in embryos, prior to replacement in the uterus at the time of IVF, should improve the chance of conception and prevent aneuploidy-related miscarriage. The reality, however, is not so straightforward and the clinical role of preimplantation genetic testing for aneuploidy (PGT-A), formerly known as preimplantation genetic screening, of IVF embryos remains uncertain with only limited evidence of effectiveness.
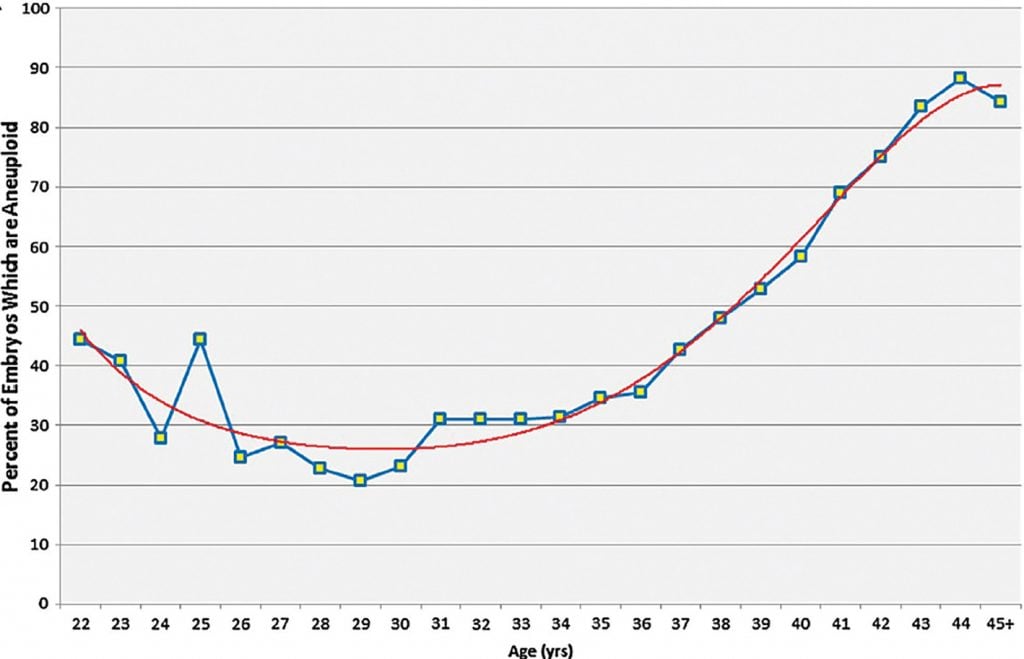
Figure 1. The rate of embryo aneuploidy relative to maternal age.
What is involved?
The technique itself is straightforward and well established. The embryos are grown for five days to the blastocyst stage, where the inner cell mass (from which the fetus is derived) is clearly separate from the outer trophectoderm (from which the membrane and placenta are derived). Following laser-assisted hatching of the outer zona pellucida, a few cells are aspirated from the trophectoderm layer of the blastocyst (Figure 2).
The DNA is extracted from these cells and amplified using polymerase chain reaction (PCR). Following amplification of the DNA, the chromosomal constitution of the cells can be assessed using a number of techniques. In the early days of preimplantation genetic testing (PGT), the original technique used was fluorescence in situ hybridisation (FISH) which produced images of five of the high-risk chromosomes. Sadly, it is now clear that this was a flawed technique and clinical studies of the effectiveness of PGT based on FISH are only of historical value.2
The next technique adopted for DNA analysis was array comparative genomic hybridisation (aCGH). This technique, which utilised a hybridisation of the embryonic DNA to an array platform containing various probes, enabled, for the first time, all 24 of the chromosomes to be assessed. However, aCGH, only has a limited capacity to distinguish between complete aneuploidy and embryonic mosaicism, a state where the result is mixed; i.e. some of the cells are euploid while others appear to be aneuploid. This limitation in detecting mosaic embryos may also be a contributing factor to the relatively high rate of miscarriage following aCGH-based PGT.3The newer technique of next-generation sequencing (NGS) gives a much clearer distinction between full aneuploidy and a mosaic embryo. In this technique, the total DNA is quantified for each of the chromosomes using parallel sequencing of multiple small DNA fragments, allowing high resolution of chromosomal abnormality detection (Figure 3). Importantly, NGS can more effectively identify mosaicism.4
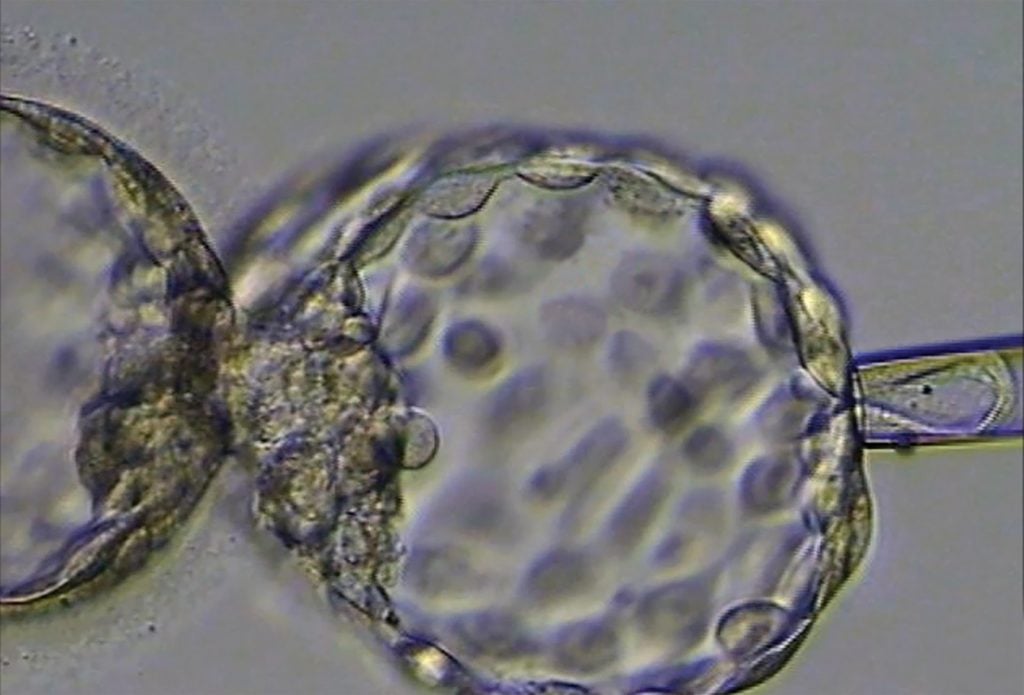
Figure 2. An embryo being biopsied for preimplantation genetic testing.
Many mosaic embryos are suitable for transfer and can lead to a healthy child, albeit with a lower success rate.5The chromosome involved in the mosaicism, along with the proportion of affected chromosome (segmental versus whole chromosome mosaicism) is critical and these cases are normally handled with expert clinical genetic support. Where a pregnancy results from a mosaic embryo, an amniocentesis should be performed to confirm the fetal karyotype.
For couples with translocations, NGS can identify unbalanced translocations in the embryo but cannot distinguish between a balanced and a fully euploid embryo. For individual genes or small chromosomal rearrangements from a preimplantation embryo biopsy, a more complex molecular technique such as karyomapping is required.6
What are the drawbacks?
There are a number of drawbacks. The removal of cells from the outside of the embryo may affect the implantation potential of the embryo.7 During the biopsy, the embryologist carrying out the procedure has to ensure that only trophectoderm (TE) is biopsied and not the inner cell mass (ICM) of the embryo (Figure 2).
Non-invasive preimplantation genetic testing, where free embryonic DNA from the culture medium is analysed, has the potential to avoid embryonic biopsy. This technique is highly promising but is not yet reliable enough for clinical use.
There are also growing concerns that PGT-A may over-diagnose aneuploidy in the fetus itself, limiting the number of embryos available for transfer.8
While PGT is clearly expensive, the overall health economics, taking into account the avoided unsuccessful transfers, may be quite reasonable.9
What is the evidence for its effectiveness?
A number of studies have demonstrated an improved implantation rate per embryo following PGT-A.10This is not surprising though, as embryos selected on the basis of having a euploid karyotype are, inevitably, going to be more likely to implant than a population of unselected embryos. The more important endpoint is the cumulative livebirth rate from one egg collection, but here studies are very limited.
One randomised controlled trial, the STAR study,11 compared the success rate per egg collection from transfer of a morphologically selected embryo against a PGT selected embryo but did not find any difference in the livebirth rate. In addition, many patients in the PGT-A group did not have any embryos to transfer, suggesting an overall reduction in the cumulative livebirth rate with PGT-A.
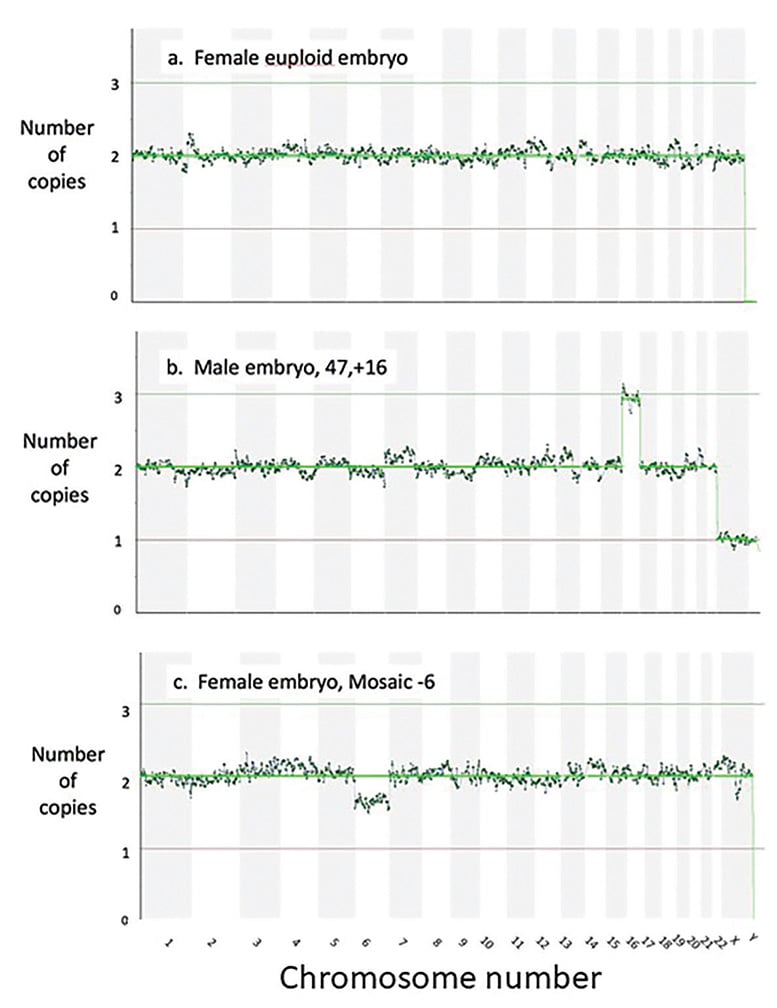
Figure 3. The readouts after next generation sequencing, showing the chromosome count for each chromosome for (a) a female euploid embryo (b) a male aneuploid embryo with an extra Chromosome 16 and (c) a female embryo mosaic for a missing Chromosome 6.
What is its current clinical role?
Some clinics, particularly in the US, routinely perform PGT-A on all embryos prior to transfer. This is not widespread practice in Australia, where PGT-A is normally used more selectively. The sort of patients who may consider PGT-A would include older women who are at higher risk of having aneuploid embryos (Figure 1) or couples who have previously gone through the traumas of a late diagnosis of an aneuploid pregnancy. Miscarriage is more complex, as most are a consequence of a chromosomal variation in the embryo, PGT-A may reduce this risk. However, studies in patients with recurrent miscarriage have not found that PGT-A is effective at reducing miscarriage risk in this population.12
What ethical issues are raised?
PGT for aneuploidy has been questioned as a form of ‘designer babies’. The technique, however, does not identify individual genes and its value lies in preventing the transfer of non-viable embryos to avoid the trauma of an unsuccessful transfer or a potentially avoidable miscarriage. Nonetheless, the technique has attracted criticism from the intersex community for discriminating against intersex people through the potential for elimination of karyotypes such as 45,XO and 47,XXY, which are consistent with a healthy life.13 It is therefore critical to avoid the judgemental language of ‘normal’ and ‘abnormal’ in this context.
How should a pregnancy be managed after PGT-A?
Regardless of whether PGT-A has been done, the routine process for prenatal screening for aneuploidy should still be followed. It is important to be aware that PGT-A cannot identify individual genes or tiny chromosomal deletions such as microdeletions.
Conclusion
In summary, in Australia, very few IVF clinics will have a policy of routine PGT-A for all embryos as the effectiveness of the intervention is still uncertain. However, most fertility specialists will offer PGT-A to selected patients following careful discussion of the pros and cons of the approach.
References
- Franasiak JM, Forman EJ, Hong KH, et al. The nature of aneuploidy with increasing age of the female partner: a review of 15,169 consecutive trophectoderm biopsies evaluated with comprehensive chromosomal screening. Fertil Steril. 2014;101(3):656-63.e1.
- Coulam CB, Jeyendran RS, Fiddler M, Pergament E. Discordance among blastomeres renders preimplantation genetic diagnosis for aneuploidy ineffective. J Assist Reprod Genet. 2007;24(1):37-41.
- Munné S, Grifo J, Wells D. Mosaicism: “survival of the fittest” versus “no embryo left behind.” Fertil Steril. 2016;105(5):1146-9.
- Munné S, Wells D. Detection of mosaicism at blastocyst stage with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;107(5):1085-91.
- Munné S, Blazek J, Large M, et al. Detailed investigation into the cytogenetic constitution and pregnancy outcome of replacing mosaic blastocysts detected with the use of high-resolution next-generation sequencing. Fertil Steril. 2017;108(1):62-71.e8.
- Gould RL, Griffin DK. Karyomapping and how is it improving preimplantation genetics? Expert Rev Mol Diagn. 2017;17(6):611-21.
- Kang H-J, Melnick AP, Stewart JD, et al. Preimplantation genetic screening: who benefits? Fertil Steril. 2016;106(3):597-602.
- Paulson RJ. Hidden in plain sight: the overstated benefits and underestimated losses of potential implantations associated with advertised PGT-A success rates. Hum Reprod. 2020;35(3):490-3.
- Lee E, Costello MF, Botha WC, et al. A cost-effectiveness analysis of preimplantation genetic testing for aneuploidy (PGT-A) for up to three complete assisted reproductive technology cycles in women of advanced maternal age. ANZJOG. 2019;59(4):573-9.
- Lee E, Illingworth P, Wilton L, Chambers GM. The clinical effectiveness of preimplantation genetic diagnosis for aneuploidy in all 24 chromosomes (PGD-A): systematic review. Hum Reprod. 2015;30(2):473-83.
- Munné S, Kaplan B, Frattarelli JL, et al. Preimplantation genetic testing for aneuploidy versus morphology as selection criteria for single frozen-thawed embryo transfer in good-prognosis patients: a multicenter randomized clinical trial. Fertil Steril. 2019;112(6):1071-9.e7.
- Sato T, Sugiura-Ogasawara M, Ozawa F, et al. Preimplantation genetic testing for aneuploidy: a comparison of live birth rates in patients with recurrent pregnancy loss due to embryonic aneuploidy or recurrent implantation failure. Hum Reprod. 2019;34(12):2340–8.
- Sparrow R. Gender Eugenics? The Ethics of PGD for Intersex Conditions. Am J Bioeth. 2013;13(10):29-38.







Leave a Reply