Preeclampsia is the most common serious medical disorder of human pregnancy.
It remains a common cause of maternal and perinatal morbidity and mortality both nationally and internationally.
In the absence of a clear and specific molecular or genetic aetiology, the definition of preeclampsia remains a syndromal one, involving an agreed upon collection of clinical features. These are pregnancy-induced hypertension (BP ≥140/90) accompanied by at least one of the following new-onset conditions at or after 20 weeks of pregnancy:
- proteinuria
- maternal organ dysfunction (eg. renal, hepatic, neurological and hematological complications)
- and/or uteroplacental dysfunction (eg. fetal growth restriction)1
A major historical justification for undertaking antenatal care (and one which remains relevant today) has been screening for preeclampsia by measuring blood pressure at routine clinical visits. This approach facilitates the eventual diagnosis of preeclampsia but does not lend itself to predicting or preventing the condition.
Recent advances in early pregnancy multiparametric screening technology and the discovery of circulating placental factors involved in the pathogenesis of preeclampsia now offer opportunities to correct this shortcoming and allow a major pregnancy care recalibration in favour of preeclampsia prediction (and thus prevention) rather than diagnosis.
The placental circulating factors are:
- soluble fms-like tyrosine kinase 1 [sFlt-1] (which increases in the maternal blood stream in advance of the clinical appearance of preeclampsia), and
- placental growth factor [PlGF] (which decreases in the maternal blood stream in advance of the clinical appearance of preeclampsia).2 3
These molecules can be measured in plasma and serum on automated laboratory platforms and are usually reported as a ratio of sFlt-1/PlGF (herein called the PreEclampsia RaTio or PERT test).
While acknowledging that these new preeclampsia prediction technologies are not yet widely available in Australia and New Zealand, their description in this article is aimed at providing some helpful background information in anticipation of their increased availability in the near future.
Early pregnancy prediction
Late first trimester screening from 11–14 weeks’ gestation using the Fetal Medicine Foundation (FMF) algorithm (derived from maternal history, mean arterial pressure [MAP], uterine artery pulsatility index [UTPI] and serum PlGF [or PAPP-A when PlGF is not available]) allows for significantly better early detection of pregnant women at increased risk of later pregnancy preeclampsia than relying on maternal risk factor assessment alone (see Table 1), though such clinical assessment is helpful in selection of cases for early screening.4 5
Table 1. Risk factors for preeclampsia.
| A past history of placental-dysfunction-related disease, including preeclampsia, fetal growth restriction and placental abruption |
| Chronic hypertension |
| Chronic kidney disease |
| Multiple pregnancy |
| Type I or II diabetes mellitus |
| Systemic lupus erythematosus/other autoimmune disease |
| Advanced maternal age (over 40 years) |
| Pregnancy interval of more than 10 years |
| First pregnancy |
| History of thrombophilia |
| Family history of preeclampsia |
| New paternity |
| Obesity |
Importantly, this early identification of women considered to be at high risk of developing preeclampsia allows them to receive prophylaxis with low dose aspirin (eg. 150 mg daily, taken at night) in a sufficiently timely fashion to significantly reduce their risk of disease, particularly early onset and preterm preeclampsia.6 7
Mid-pregnancy prediction
Second trimester (19–24 weeks) screening using the FMF algorithm has also been demonstrated in an Australian population to be effective in identification of women at risk of preterm preeclampsia.8 While detection of this group may be too late for full prophylactic efficacy of low dose aspirin, increased pregnancy care surveillance and use of the PERT test (see below) allows for appropriate clinical vigilance as their pregnancies progress.
Later pregnancy prediction
In the latter half of pregnancy, increasing evidence is demonstrating the clinical utility of the PERT test in the prediction, diagnosis, differential diagnosis and management of preeclampsia.
In the PROGNOSIS trial, which studied 1050 women with suspected preeclampsia, a PERT result of ≤38 ruled out preeclampsia within one week with a negative predictive value of 99.3% and within four weeks with a negative predictive value of 94.3%. A PERT result >38 ruled in preeclampsia within four weeks with a positive predictive value of 36.7%.9
Similar results have been reported in other studies involving different populations, and the PERT test has also been shown to assist triage towards appropriate hospitalisation in women with suspected preeclampsia (references available on request).
These studies support the use of the PERT test for stratifying pregnant women with suspected preeclampsia into a low-risk group who can safely continue with routine outpatient antenatal care and a high-risk group who require increased maternal and fetal welfare surveillance, possibly as inpatients. Such stratification optimises patient satisfaction and cost-effective use of hospital resources.
An example of a clinical practice guideline for use in a pregnancy day care setting when performing the PERT test is provided in Figure 1, and Table 2 provides some illustrative case scenarios.
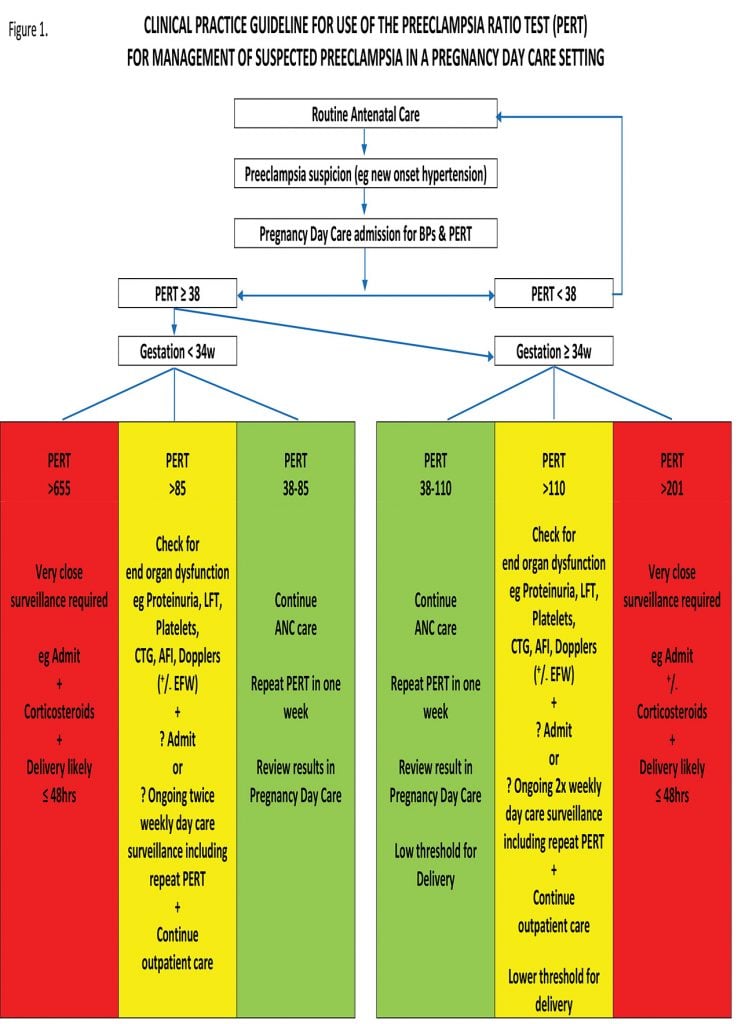
Figure 1. Clinical practice guideline for the use of the preeclapsia ratio test (PERT)
Table 2. Illustrative case scenarios
| Case 1: 26 yo, G1P1, BMI 25 Pregnancy -> uneventful until 33/40; c/o increased oedema; BP 135/85, urinalysis = nad; PERT 145; increased antenatal surveillance (twice weekly short stay pregnancy day care reviews); PE diagnosed at 35/40; delivery at 36/40 for worsening PE. |
| Case 2: 33 yo, G2P2, BMI 27 First pregnancy -> delivery at 30/40 with severe PE and FGR. Second pregnancy -> much anxiety; LDA from 10/40; monthly PERT testing from 22/40; All PERTs < 38; anxiety much relieved; outpatient antenatal care only; -> delivery at term. |
| Case 3: 35 yo, GIP1, BMI 38 Pregnancy -> GDM at 28/40; borderline HT 140/85 at 31+1/40; PERT = 902!; urgent scan -> previously unsuspected severe FGR (<3rd percentile); immediate admission, corticosteroid therapy; delivery at 31+3; BW = 1130 g; baby discharged home after five weeks of neonatal care weighing 2274 g. |
| Case 4: 40 yo, G1P1, BMI 30; IgA nephropathy; LDA from 12/40 Pregnancy -> persistently borderline HT and urinalysis ++/+++ throughout pregnancy; monthly PERT testing from 20/40; All PERTs < 38; no superimposed PE; delivery at term. |
Local and international experience with the PERT test also supports its value:
- in the surveillance of asymptomatic women at high risk of preeclampsia. For example, women who have a predicted high risk for preeclampsia on history or using the FMF late first trimester algorithm may be followed with monthly PERT tests from 20 weeks, even if they are asymptomatic. Under these circumstances, a PERT test result of <38 is very reassuring for both the patient and her clinician.
- in assessing the severity of preeclampsia. The higher the PERT result, the higher the risk of maternal complications such as acute lung oedema, HELLP syndrome, placental abruption, renal failure or refractory hypertension. The PERT test can also be useful in predicting fetal outcomes related to growth and prematurity of the baby.
- in assessing the rate of progression of preeclampsia. The rate of change of PERT levels is an important marker for the progression of disease. A rapid increase in PERT results points to a worsening of the clinical situation, including earlier onset disease, placental dysfunction associated with fetal growth restriction, a shorter interval to delivery and more adverse outcomes, although this does not necessarily mean that immediate delivery is always necessary.
- in the differential diagnosis of conditions which mimic preeclampsia, including non-HELLP thrombocytopenia, chronic hypertension, chronic kidney disease (eg. lupus nephritis, diabetic nephropathy, renal transplant), migraine headache or other types of chronic headache, obese patients with fatty liver of pregnancy who may have transaminitis.
- in predicting the risk of adverse outcomes in high-risk pregnancies with comorbidities such as autoimmune diseases (systemic lupus erythematosus, the anti-phospholipid syndrome) and diabetes. The PERT test can discriminate between placental dysfunction and other complications related to comorbid disease (eg. lupus-flare, lupus nephritis).
- as a superior predictor of preeclampsia compared to other biochemical (eg. uric acid, liver enzymes, urinary protein) and haematological (eg. haemoglobin, platelets) tests.
- in twin pregnancies, which carry twice the risk of developing preeclampsia compared to singleton pregnancies. While the PERT test provides prognostic and diagnostic information in such pregnancies, variations in the PERT normal ranges between singleton and twin pregnancies mean the use of the ratio test beyond 30–32 weeks is less discriminatory for preeclampsia in twin pregnancies than when used in singletons.
- in stratifying small for gestational age fetuses into healthy small babies and those with fetal growth restriction secondary to chronic placental insufficiency.
- for management planning and decision making relating to delivery timing.
- as a cost-effective adjunct for the clinical management of preeclampsia in a range of healthcare settings.
- as a possible point-of-care test in low- to middle-income health care settings to identify those pregnant women who can safely remain in their local environments and those who need transfer to higher levels of maternity care.
- in the selection of participants for clinical trials of preeclampsia management by identifying patients at higher risk of developing preeclampsia and thereby facilitating more cost-effective study recruitments of sufficient sample sizes.
Conclusion
New technologies for identifying women at risk of developing preeclampsia promise to facilitate improved maternal and perinatal outcomes in this high-risk group. Just as importantly, these technologies will also allow women identified as low risk to safely minimise unnecessary interventions during their pregnancies.
References
- Brown MA, Magee LA, Kenny LC, et al. Hypertensive disorders of pregnancy: ISSHP classification, diagnosis, and management recommendations for international practice. Hypertension. 2018;72(1):24–43.
- Maynard SE, Min JY, Merchan J, et al. Excess placental soluble fms-like tyrosine kinase 1 (sFlt1) may contribute to endothelial dysfunction, hypertension, and proteinuria in preeclampsia. J Clin Invest. 2003;111(5):649-658.
- Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350(7):672–683.
- Fetal Medicine Foundation. Risk for preeclampsia. 2021. Available from: https://fetalmedicine.org/research/assess/preeclampsia/first-trimester. Accessed 05 February 2021.
- Park FJ, Leung CH, Poon LC, et al. Clinical evaluation of a first trimester algorithm predicting the risk of hypertensive disease of pregnancy. ANZJZOG. 2013;53(6):532-9
- Rolnik DL, Wright D, Poon LC, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med. 2017;377(7):613–22.
- Park F, Russo K, Williams P, et al. Prediction and prevention of early-onset pre-eclampsia: impact of aspirin after first-trimester screening. Ultrasound Obstet Gynecol. 2015;46(4):419-23
- Black C, Rolnik DL, Al-Amin A, et al. Prediction of preterm pre-eclampsia at midpregnancy using a multivariable screening algorithm. ANZJZOG. 2020;60(5):675-82.
- Zeisler H, Llurba E, Chantraine F, et al. Predictive value of the sFlt-1:PlGF ratio in women with suspected preeclampsia. N Engl J Med. 2016;374(1):13–22.






Predicting preeclampsia early can significantly improve pregnancy outcomes. Advanced screening methods are vital for proactive management and care.