Introduction
Neonates are vulnerable to bacterial sepsis, a condition recognised for its rapid progression. Preventing sepsis-related complications and mortality necessitates early, empirical treatment of neonates based on risk factors before severe symptoms manifest. This differs from other areas of clinical medicine, where treatment typically begins after confirming a diagnosis. The prevailing approach is to treat when there is a reasonable suspicion of sepsis and discontinue antibiotics if sepsis is ruled out. This approach underscores the fear associated with unrecognised neonatal sepsis. Notably, clinicians tend to have a low threshold for treatment, leading to frequent overuse of antibiotics. Over the past decade, a significant shift has occurred in the management of early-onset sepsis (EOS) in infants born after 35 weeks of gestation. This shift involves the adoption of the Kaiser Permanente newborn early-onset sepsis calculator,¹ replacing categorical algorithms.
Early-onset sepsis
This article focuses on EOS, defined as neonatal sepsis within the first 48–72 hours after birth. The primary causative organisms are group B streptococcus (GBS) and Escherichia coli (E. coli). EOS transmission typically occurs during childbirth, with an incidence ranging from 0.3 to three cases per 1000 live births.2,3 In the most extensive study to date, encompassing 757,979 infants born between 2014–2018 in Europe, North America and Australia, 2.86% of all live births, approximately one in 35, received antibiotics for suspected EOS.⁴ However, the incidence of EOS was only 0.49 cases per 1000 live births in the study population. Notably, there was also a wide variation in antibiotic use among study centres, ranging from 1.2% to 12.5%.
Early-onset sepsis calculator
Escobar et al. used maternal data and clinical neonatal findings from 608,014 live births to develop a predictive model to estimate the risk of EOS and stratify neonatal management.⁵ This calculator is available online at neonatalsepsiscalculator.kaiserpermanente.org.
A validation study of the calculator at a Kaiser Permanente Northern California hospital showed that there was a decrease in antibiotic use from 5% to 2.6% without adverse events.⁶ Similarly, implementation of the EOS calculator in Western Australia saw a reduction in antibiotic use from 12% to 7.6%.⁷ Perinatal units across Australia increasingly use this calculator to stratify the management of newborns rather than algorithmic flow charts that were previously used to guide decision-making.
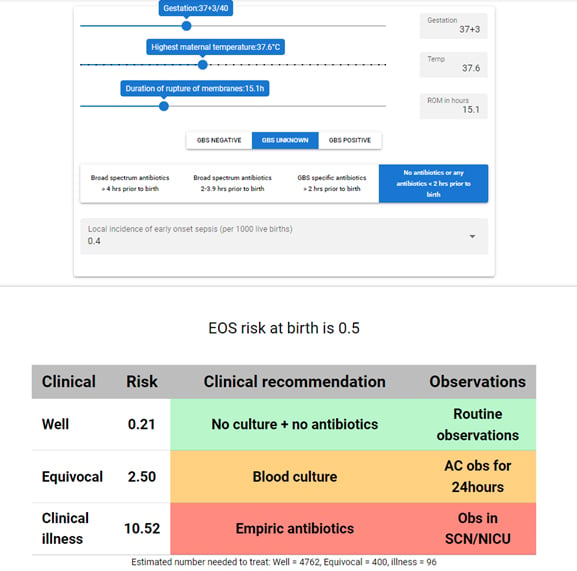
Figure 1. Example early-onset sepsis risk calculation (available at https://sepsiscalc.org).8
Determinants used to guide EOS risk stratification
Figure 1, from sepsiscalc.org (a localised implementation based on the regression equations of the Kaiser Permanente EOS sepsis calculator), shows the various factors used to determine EOS risk. These ‘inputs’ into the calculator are as follows:
- Gestation at birth of the infant.
- Highest maternal antepartum temperature.
- Duration of rupture of membranes.
- Maternal GBS status and maternal antibiotic administration.
- Local incidence of sepsis: a site-specific baseline risk of EOS. A risk of 0.4 per 1000 live births is used at the Royal Women’s Hospital in Melbourne.
The above input determines the EOS risk of birth, shown as 0.5 in Figure 1. A further composite risk is then determined by incorporating the neonate’s clinical presentation in the first 24 hours. The clinical presentation is classified as one of the following:
- Well appearing: infant with no persistent physiological abnormalities.
- Equivocal: infant with a single physiological abnormality lasting >4 hours (or two lasting >2 hours), such as tachycardia ≥160, or tachypnoea ≥60, temperature instability or respiratory distress.
- Clinical illness: infant requiring respiratory support, showing haemodynamic instability or presenting evidence of neonatal encephalopathy.
This approach to EOS risk stratification allows for clearer clinical recommendations, using a traffic-light color-coding style. Recommendations are based on the composite risk score:
- Composite EOS risk <1 per 1000 (Green): no blood culture or antibiotics required. Additional observations only required if EOS risk at birth is >1 per 1000.
- Composite EOS risk 1 to ≤3 per 1000 (Amber): the recommendation is that blood culture is collected but no antibiotics are instituted.
- Composite score of ≥3 (Red): empiric antibiotic treatment is recommended with admission to special care of newborn intensive care unit.
It should be noted that, irrespective of composite score, the calculator will always recommend empiric antibiotics should the infant have clinical illness.
Empiric treatment
For infants requiring empiric antibiotics, the mainstay of treatment is intravenous benzylpenicillin and gentamicin, given that GBS and E. coli are the most common pathogens. Increased attention has also been placed on early cessation of antibiotics (ie at 36 hours) if the blood culture remains negative and the neonate remains well.
Areas of controversy
The practice of obtaining a blood culture from a neonate without immediately starting intravenous antibiotics is novel for neonatologists. In the past, it was customary that if a neonate was deemed at risk enough to undergo a blood culture, they should also receive empiric antibiotics right away. Critics of the new approach contend that following the calculator’s guidelines might result in delays in initiating antibiotic treatment in cases where the blood culture eventually reveals a positive result. In contrast, the authors of the calculator argue that these trade-offs are rooted in statistical data and therefore are justifiable.⁹
A meta-analysis to assess the sensitivity of the calculator prior to implementation in the United Kingdom compared the existing National Institute for Health and Care Excellence (NICE) guidelines to the EOS calculator approach and noted that in the subset of babies exposed to chorioamnionitis the calculator might be more likely to miss cases.10 The authors of the calculator state that one of their explicit goals was to estimate risk without use of the obstetric diagnosis of chorioamnionitis, which can be subjective (prior to histopathology). The calculator is intended to capture objective measures that correlate with chorioamnionitis, but the authors concede that in cases such as maternal bacteraemia or hypotension the newborn would warrant treatment irrespective of risk estimates.
Conclusion
Recognising the pivotal role of antepartum factors in risk assessment, effective management of early-onset neonatal sepsis necessitates close collaboration among midwives, obstetricians and paediatric teams. This collaborative approach facilitates the timely identification of potential risk factors and enables clinical assessment of newborns within the context of these risk factors. Treatment strategies involve the early administration of antibiotics for clinically unwell infants and more judicious use of antibiotics in cases where there is significant risk. Transitioning from broad algorithmic guidelines to individualised, data-driven recommendations will help minimise unnecessary treatment. Furthermore, vigilant observational care will assume increasing importance in identifying infants who might not be adequately captured by the risk calculators as antibiotics use rightfully becomes increasingly judicious.
References
- Kaiser Permanente Research. Neonatal early-onset sepsis calculator. 2003 [cited 29 October 2023]. Available at neonatalsepsiscalculator.kaiserpermanente.org/InfectionProbabilityCalculator.aspx
- Fleischmann C, Reichert F, Cassini A, et al. Global incidence and mortality of neonatal sepsis: a systematic review and meta-analysis. Arch Dis Child 2021;106(8):745–752.
- Braye K, Foureur M, de Waal K, Jones M, Putt E, Ferguson J. Epidemiology of neonatal early-onset sepsis in a geographically diverse Australian health district 2006–2016. PLoS One 2019;14(4):e0214298. doi:10.1371/journal.pone.0214298
- Giannoni E, Dimopoulou V, Klingenberg C, et al. Analysis of antibiotic exposure and early-onset neonatal sepsis in Europe, North America, and Australia. JAMA Netw Open 2022;5(11):e2243691. doi:10.1001/jamanetworkopen.2022.43691
- Escobar GJ, Puopolo KM, Wi S, et al. Stratification of risk of early-onset sepsis in newborns ≥ 34 weeks’ gestation. Pediatrics 2014;133(1):30–36. doi:10.1542/peds.2013-1689
- Kuzniewicz MW, Puopolo KM, Fischer A, et al. A quantitative, risk-based approach to the management of neonatal early-onset sepsis. JAMA Pediatr 2017;171(4):365–371. doi:10.1001/jamapediatrics.2016.4678
- Strunk T, Buchiboyina A, Sharp M, Nathan E, Doherty D, Patole S. Implementation of the Neonatal Sepsis Calculator in an Australian Tertiary Perinatal Centre. Neonatology 2018;113(4):379–382. doi:10.1159/000487298
- John J. Neonatal sepsis calculator. 2021 [cited 29 October 2023]. Available at sepsiscalc.org






Leave a Reply