Metabolic requirements dramatically increase in pregnancy and thyroid hormone dosing needs to increase proportionately. Insufficient thyroxine replacement can have serious affects on the growing fetus, including increased risk of premature birth, low birthweight and miscarriage.1 These affects are seen especially in the first trimester, as the fetus is relying solely on transplacental free T3 and T4 until it develops its own thyroid gland at 11–12 weeks gestation. Furthermore, the fetal thyroid does not fully mature and secrete adequate thyroid hormone until 16 weeks.2 The first trimester of pregnancy tends to be managed entirely by general practitioners, necessitating clear guidelines for investigation, treatment and monitoring of hypothyroidism in such a critical trimester.
Thyroid physiology undergoes many changes during pregnancy. In the first trimester, beta HCG rises. TSH and HCG share similar beta subunits, allowing the HCG to directly stimulate thyroid tissue, this leads to increased fT3 and fT4 and, therefore, TSH suppression may occur in the first trimester. As beta HCG falls in the second and third trimester, TSH normalises. Furthermore, there is:
- increased iodine clearance through increased glomerular filtration and decreased tubular reabsorption;
- increased uptake of T3 and T4;
- increased oestrogen, causing increased thyroxine-binding globulin (TBG) production in the liver, reducing biologically active fT4;
- T4 undergoes transplacental passage to supply the fetus;
- increased renal excretion of T4; and
- breakdown of T4 by placental deiodinases
In view of these physiological changes, gestation-specific thyroid stimulating hormone (TSH) level standards need to be consulted when interpreting thyroid function tests, as they are different to non-pregnant interval ranges.3
Universal screening for thyroid dysfunction in pregnancy is not recommended4; however, thyroid function testing is recommended by the American Thyroid Association in the woman5:
- from an area with moderate to severe iodine insufficiency;
- with symptoms of hypothyroidism;
- with a family or personal history of thyroid disease;
- with a personal history of thyroid peroxidase antibodies;
- who has type 1 diabetes;
- who has had head and neck radiation;
- who has experienced recurrent miscarriage/reduced fertility; or
- with morbid obesity.
Hypothyroidism may be pre-existing (Hashimoto’s thyroiditis, iodine deficiency, previous radiation exposure to the thyroid, previous thyroidectomy or thyroid nodule excision) or newly diagnosed in pregnancy. It can be divided into either:
- overt hypothyroidism (OH) (affecting 0.5 to one per cent of all pregnancies);
- subclinical hypothyroidism (SCH) (two to 15 per cent of all pregnancies). The incidence varies widely across studies depending on which value of TSH is used as the upper limit of normal.6 7
- Pregnancy causes changes in thyroid hormone metabolism.
- Thyroid function screening is suggested in at-risk women, to avoid the detrimental affect on both maternal and fetal outcomes.
- Studies are ongoing to determine if universal screening should be recommended.
- SCH and OH in pregnancy require monitoring.
- Dietary iodine supplementation should begin at diagnosis of pregnancy.
- A prompt increase in thyroxine dose in OH will decrease adverse events to both mother and fetus.
- Treatment of SCH in pregnancy is controversial; however, the European Thyroid Association recommends treatment. Studies are ongoing and will soon provide definitive evidence.
Treatment and surveillance
For the treatment of OH or SCH in pregnancy, the optimal agent is oral T4. The goal is to normalise the maternal serum TSH to within the normal trimester-specific pregnancy reference ranges. Thyroxine should be administered first thing in the morning, before food, and at least half an hour before other medications.8 Patients should be advised to avoid taking drugs that may impair absorption directly post-thyroxine.9 These include10 :
- iron;
- calcium-containing supplements; and
- proton pump inhibitors.
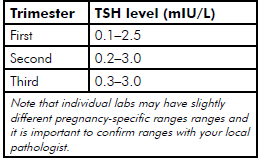
Table 1. Suggested target TSH levels specific to gestation.
Iodine supplementation
Pregnant women are recommended to ingest 220µg of iodine per day; women who are breastfeeding are recommended to ingest 270µg per day.11 In 2007, a study demonstrated that Australian women are currently mildly iodine deficient, with a mean intake of 100µg per day.12 Therefore, the recommendation is to take a supplement containing 150µg of iodine each day. This dose is safe and effective for pregnant and breastfeeding women. Pregnant women in search of vitamin and mineral supplements should be advised to check that the formulation includes the recommended amount of iodine.
Newly diagnosed OH
The incidental finding of OH during pregnancy screening should warrant immediate T4 replacement. A recent study suggested the average daily dosing of thyroxine in OH should be approximately 2.3µg per kilogram of body weight.13 Despite this recent study the usual commencing dose of T4 for newly diagnosed OH would be 100–150µg per day.14 15 The TSH should then be revaluated within four weeks, and dose adjustments made appropriately. The diagnosis of OH during the perinatal period should warrant further enquiry:
- focus on patient history, screeningfor previous thyroid surgery,
- for treatment with radioactive iodine for previous Graves’ disease, dietary iodine deficiency, drug induced hypothyroidism, and other autoimmune disease (particularly type 1 diabetes); examine for signs of hypothyroidism, which can affect the cardiovascular, neurologic and musculoskeletal system; and
- investigate for presence of thyroid autoantibodies (anti-TPO and anti-thyroglobulin antibodies, and thyroid receptor antibodies if there is a history of treated Graves’ disease) and ongoing thyroid function test monitoring.
Pre-existing diagnosis of OH
There is an increased need for thyroxine replacement in women with pre-existing OH, owing to the normal physiology of pregnancy. In those with pre-existing OH, the thyroxine dose should be increased by 20–40 per cent from very early gestation.16
Currently, guidelines recommend optimisation of maternal TSH in the preconception period. After conception, an increase in T4 as soon as possible with the goal of normalising TSH is recommended. An easy approach is to increase total T4 dose by two tablets per week or by 20–40 per cent of baseline when pregnancy is diagnosed.17 18
The presence of thyroid-stimulating receptor antibodies (typical of Graves’ disease) in a patient treated with complete thyroidectomy or radioactive iodine now requiring thyroxine replacement for treatment of their resultant hypothyroidism, will require specialist input. The persistent circulating maternal TSH receptor antibodies can cross the placenta and potentially have deleterious effects on the fetus, resulting in fetal hyperthyroidism. This could potentially cause fetal anaemia and heart failure. This condition warrants a notification to the obstetrician and referral either to an endocrinologist or to an obstetric medicine physician.
Subclinical hypothyroidism
Approximately 50–60 per cent of women with SCH will have positive antibodies and therefore all women with SCH must be tested for their presence. There are currently discrepancies in the guidelines relating to the treatment of subclinical hypothyroidism, and the role of T4 in reducing adverse outcomes is unclear.19 However, the European Thyroid Association recommends treating all SCH women (TPO antibody positive and negative) with T4.20 One approach would be to commence between 50–75µg per day T4 and then retest the thyroid function in four weeks.21 A recent study which included only 77 women with SCH suggested slightly higher dosing may be needed.22
| Type | Lab diagnosis | Risks |
| Overt hypothyroidism | ↑TSH and ↓ fT4 OR TSH >10 (irrespective of fT4) | Fetal: prematurity, low birthweight, perinatal mortality, cognitive impairment and developmental delay 23
Maternal: anovulation, miscarriage risk, increased gestational hypertension, anaemia, postpartum haemorrhage24 |
| Subclinical hypothyroidism and thyroid antibody negative | ↑TSH and normal fT4 and no auto-thyroid antibodies | Higher risk of pregnancy loss, placental abruption, premature rupture of membranes, and neonatal death25
Treatment advised by the European Thyroid Association26, though unclear the value of T4 treatment in preventing adverse outcomes27 |
| Subclinical hypothyroidism plus TPO antibody positive | ↑TSH and normal fT4 | A single study demonstrated reduction of miscarriage and preterm birth with thyroxine treatment 28 |
Table 2. Diagnosis and risks associated with subtypes of hypothyroidism in pregnancy.
Monitoring
In those with treated OH, serum TSH should be monitored regularly in the first trimester to ensure the patient is euthyroid, and then at least once in the second and third trimesters.29 The thyroid function tests should be re-checked four weeks after any dosage adjustments in thyroxine, to ensure the patient is euthyroid. Following delivery, the T4 dose should be reduced to the patient’s preconception dose if they had been euthyroid on that dosage, with a follow-up TSH measurement approximately four to six weeks postpartum.30
Women with SCH in pregnancy who are not initially treated should be monitored throughout their pregnancy to detect progression to OH. Currently, guidelines suggest this should take place every four weeks during the first trimester of pregnancy, and then at least once in the second and third trimesters.31 In the postpartum period we would usually cease therapy and recheck thyroid function tests four weeks later. If, however, the TSH was significantly elevated we would consider a more gradual reduction in T4 to ovoid OH, with repeat thyroid function tests every four weeks.
A failure to achieve a sufficient response in TSH after appropriate therapy should spur investigation into causes for lack of T4 absorption. This may represent one of the following: poor compliance; drug interactions; or impaired absorption.
References
- Abalovich M, Amino N, Barbour L et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical PracticeJ Clin Endocrinol Metab. 2007; 92(8 Suppl):S1-47.
- Obregon M, Calvo R, Del Rey F et al. Ontogenesis of thyroid function and interactions with maternal function. Endocrine Development. 2007; 10:86-98
- Abalovich M, Vazquez A, Alcaraz G et al. Adequate levothyroxine doses for the treatment of hypothyroidism newly discovered during pregnancy. 2013; 23: 1479-1483.
- Negro R, Schwartz A, Gismondi R et Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010; 95(4):1699-707.
- Stagnaro-Green A, Abalovich M, Alexander E et Guidelines of the American Thyroid Association for the Diagnosis and Management of Thyroid Disease during Pregnancy and Postpartum. Thyroid. 2011; 21:1-46.
- Lazarus JH: Thyroid function in pregnancy.Br Med Bull. 2011; 97: 137-148.
- Stagnaro-Green A. Postpartum Management of Women Begun on Levothyroxine during Pregnancy. Endocrinol. 2015; 6:183.
- Yassa L, Marqusee E, Fawcett R et Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J Clin Endocrinol Metab. 2010; 95:3234-3241.
- Liwanpo L, Hershman Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009; 23:781-92.
- Liwanpo L, Hershman Conditions and drugs interfering with thyroxine absorption. Best Pract Res Clin Endocrinol Metab. 2009; 23:781-92.
- National Health and Medical Research Council and New Zealand Ministry of Health (2006). Nutrient Reference Values for Australia and New Zealand including Recommended Dietary Intakes.
- The Royal Australian and New Zealand College of Obstetricians and Gynaecologists Statement: Testing for hypothyroidism during pregnancy with serum TSH. Current July 2015
- Abalovich M, Vazquez A, Alcaraz G et al. Adequate levothyroxine doses for the treatment of hypothyroidism newly discovered during pregnancy. 2013; 23: 1479-1483.
- Stagnaro-Green A. Postpartum Management of Women Begun on Levothyroxine during Pregnancy. Endocrinol. 2015; 6:183.
- Devdhar M, Ousman YH, Burman Hypothyroidism. Endocrinol Metab Clin N Am. 2007; 36:595-615.
- Obregon M, Calvo R, Del Rey F et al. Ontogenesis of thyroid function and interactions with maternal function. Endocrine Development. 2007; 10:86-98
- Yassa L, Marqusee E, Fawcett R et Thyroid hormone early adjustment in pregnancy (the THERAPY) trial. J Clin Endocrinol Metab. 2010; 95:3234-3241.
- Alexander E, Marqusee E, Lawrence J et al. Timing and magnitude of increases in levothyroxine requirements during pregnancy in women with hypothyroidism.N Engl J Med. 2004; 351:241-249.
- Maraka S, Ospina N, Espinosa De Ycaza A et al. Subclinical Hyothyroidism in Pregnancy: A systematic Review and Meta- Thyroid. 2016; 26(4):580-590.
- Lazarus J, Brown R, Daumerie C et al. European Thyroid Association Guidelines for the Management of Subclinical Hypothyroidism in Pregnancy and Children. Eur Thyroid. 2014; 3:76-94.
- Stagnaro-Green A. Postpartum Management of Women Begun on Levothyroxine during Pregnancy. Endocrinol. 2015; 6:183.
- Abalovich M, Vazquez A, Alcaraz G et al. Adequate levothyroxine doses for the treatment of hypothyroidism newly discovered during pregnancy. 2013; 23: 1479-1483.
- Abalovich M, Amino N, Barbour L et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical PracticeJ Clin Endocrinol Metab. 2007; 92(8 Suppl):S1-47.
- Abalovich M, Amino N, Barbour L et al. Management of thyroid dysfunction during pregnancy and postpartum: an Endocrine Society Clinical PracticeJ Clin Endocrinol Metab. 2007; 92(8 Suppl):S1-47.
- Maraka S, Ospina N, Espinosa De Ycaza A et al. Subclinical Hyothyroidism in Pregnancy: A systematic Review and Meta- Thyroid. 2016; 26(4):580-590.
- Lazarus J, Brown R, Daumerie C et al. European Thyroid Association Guidelines for the Management of Subclinical Hypothyroidism in Pregnancy and Children. Eur Thyroid. 2014; 3:76-94.
- Maraka S, Ospina N, Espinosa De Ycaza A et al. Subclinical Hyothyroidism in Pregnancy: A systematic Review and Meta- Thyroid. 2016; 26(4):580-590.
- Negro R, Schwartz A, Gismondi R et Universal screening versus case finding for detection and treatment of thyroid hormonal dysfunction during pregnancy. J Clin Endocrinol Metab. 2010; 95(4):1699-707.
- Lazarus J, Brown R, Daumerie C et al. European Thyroid Association Guidelines for the Management of Subclinical Hypothyroidism in Pregnancy and Children. Eur Thyroid. 2014; 3:76-94.
- Devdhar M, Ousman YH, Burman Hypothyroidism. Endocrinol Metab Clin N Am. 2007; 36:595-615.
- Lazarus J, Brown R, Daumerie C et al. European Thyroid Association Guidelines for the Management of Subclinical Hypothyroidism in Pregnancy and Children. Eur Thyroid. 2014; 3:76-94.






Leave a Reply