Pre-implantation genetic testing (PGT) is used within an IVF cycle. There are two types: pre-implantation genetic diagnosis (PGD) and preimplantation genetic screening (PGS). PGD is indicated for the diagnosis of genetic disease in early embryos before implantation. PGS tests embryos for euploidy (the correct number of chromosomes) before implantation. Both types of PGT are embryo-selection tools to enable couples to choose an embryo that is more likely to develop into a healthy child.
PGD was first reported by Alan Handysides in 1990, using polymerase chain reaction in order to sex embryos to prevent sex-linked disease1, and then in 19922 for cystic fibrosis that ultimately resulted in the birth of a healthy girl. Before the development of PGD, couples at risk of having an affected child could have chorionic villus sampling or amniocentesis in order to detect the genetic disease. Both procedures carry risks to the pregnancy and require parents to face the challenging decision whether to continue the pregnancy if the child is affected. PGD offered the option of screening the embryos before implantation, so parents could be confident that their child was free of the genetic illness. PGD also meant that tested embryos could be frozen for future use.
The PGD process
PGD as a process has evolved since it was first described, both in terms of the stage that embryos are biopsied and the technology used to detect the genetic issues. The woman goes through an IVF cycle in which her ovaries are stimulated to produce a number of oocytes. The oocytes are collected using a transvaginal-ultrasound-guided procedure, injected with sperm and the resulting embryos are cultured for several days. The embryos are biopsied and the cells sent to a genetics lab for analysis. Unaffected embryos are then transferred into the woman’s uterus.
PGD can be performed on the polar bodies from the oocyte – day-three embryos that typically contain six to ten cells – or from the trophectoderm of a day-five or -six blastocyst containing 50–100 cells. Polar body biopsies can be performed either before or after fertilisation. Polar bodies have no role in embryo development and so their removal is not thought to compromise the embryo’s development. Polar body biopsy also means there is a reasonable interval in which to obtain testing results before embryo transfer on day five. The big disadvantages are that it gives no information about the genetics of the sperm or mitotic errors that occur after the embryo starts growing. Today, polar body biopsy is rarely performed.
PGD was initially performed on day-three embryos at around the six to ten cell stage and one or two cells were removed. Since total culture time is five to six days before embryos must be replaced or frozen, biopsying on day three allows two or three days for results to be available before the transfer of the embryo. There were some concerns that day-three biopsies could lower the chances of a pregnancy and, in 2013, a randomised study was published confirming that biopsying embryos on day three reduced their implantation potential from 50 per cent to 30 per cent, whereas day-five trophectoderm biopsy had no negative impact.3
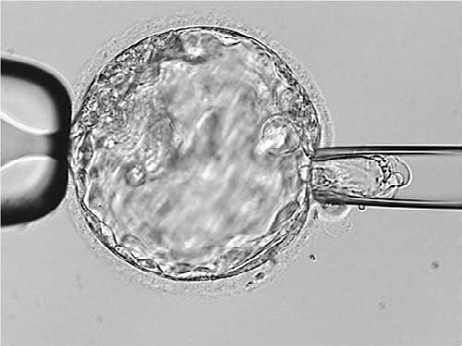
Figure 1. Biopsy of a day-five blastocy
Lowered implantation potential along with the limited amount of genetic material available for analysis means that PGD is now more commonly performed on day-five or -six embryos at the blastocyst stage. Embryo freezing technology using vitrification enables embryos to be frozen without compromising pregnancy rates while genetic testing is performed.
In order to obtain cells from the trophectoderm, a laser is used on the zona pellucida on day three of development to produce a hole that trophectoderm cells protrude through on day five or six. Once an embryo has reached the expanded blastocyst stage, a laser is used to obtain between five and ten cells. Unlike day-three biopsy, which uses only one or two cells, trophectoderm biopsy provides more cells for testing and thus improves the diagnostic accuracy of the genetic testing. Moscaism can mean that there is a discordance between cells from the inner cell mass and those obtained from the trophectoderm; however, more recent studies suggest that the majority of cells are concordant. This means that the results obtained from the trophectoderm largely reflect the genetic make-up of the inner cell mass.
What are the indications for PGD?
PGD is offered to couples who carry a genetic disease with known mutations, making it suitable for genetic analysis. The most common diseases that PGD is used for include cystic fibrosis, Huntington’s disease, spinal muscular atrophy, BRCA genes, haemophilia and parental translocations. PGD may also be used for human leukocyte antigen testing, when a baby could be a stem cell donor for a relative. There are now more than 300 monogenic conditions for which PGD has been used, with the number continuing to expand. There have been thousands of healthy children born following PGD.
Some couples choose carrier testing before starting to conceive a family. Carrier screening often occurs where there is no family or personal history of a genetic illness; however, both partners are screened to determine whether they are carriers of particular genetic diseases. Often these couples will then choose PGD if they are found to be at risk. The most common carrier screening programs test for cystic fibrosis, spinal muscular atrophy and Fragile X syndrome.
PGS
Maternal ageing is associated with increasing oocyte aneuploidy, making embryonic aneuploidy the most common reason IVF fails in older patients. PGS is often offered to older women, couples with recurrent pregnancy loss and those who have experienced failed IVF cycles.
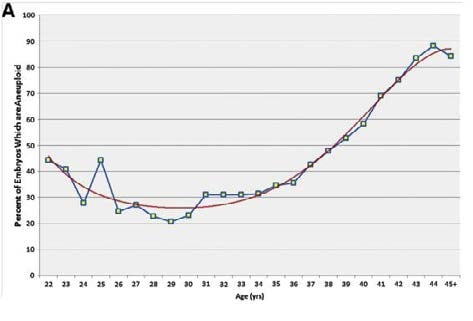
Figure 2. Aneuploidy rate by maternal age.
The first PGS cycle was reported in 19934 using fluorescent in situ hybridisation (FISH) to screen cells from day-three embryos. The major drawback of this technology was that only a limited number of FISH probes could be used simultaneously; probes were chosen to test for the chromosomes most commonly linked to miscarriage (X, Y, 13, 18, 21), thus missing the impact of aneuploidies from chromosomes not tested. A randomised controlled trial then showed no benefit with day-three PGS and, indeed, fewer babies from an IVF cycle were reported than replacing embryos based on morphological appearances.
Array comparative genomic hybridisation (aCGH) and, more recently, next-generation sequencing (NGS) allows analysis of all of the chromosomes, which, combined with trophectoderm biopsy, has made PGS an effective testing option. In a select population, livebirth rates of around 70 per cent are observed when a single euploid embryo is transferred. Though these results are promising, the challenge is that a couple may not have any embryos to biopsy or any that are euploid.
PGS is a tool for embryo selection and does not increase the number of babies obtained from an IVF cycle. PGS does, however, reduce miscarriage rates, as aneuploid embryos are not replaced, thus reducing the number of thaw cycles a patient must go through to get to a normal embryo. PGS is also being used in the USA to reduce the number of embryos being transferred, with some insurance companies funding PGS so that a single embryo is transferred, hence reducing the costs and risks associated with multiple pregnancies. PGS is also highly informative for the parents and clinician. Where IVF has been previously unsuccessful, information that all embryos turn out to be aneuploid helps some couples consider gamete donation.
Analysis of the biopsied cells
The most common technology used to analyse the cells for PGS is aCGH. The DNA is extracted, then amplified and labelled with a fluorescent green label. The reference DNA is also amplified and labelled with a red label. Both the test and reference DNA are then hybridised on to normal metaphase chromosomes. The green/red ratio is analysed to determine the number of chromosomes. The use of aCGH has some drawbacks, as it does not detect triploidy or balanced translocations.
Single gene disorders are mostly detected using single-cell multiplex PCR. Karyomapping is a new technique being developed where single nucleotide polymorphisms (SNP) can be analysed within the informative loci using aCGH,without the need for the development of costly probes.
Many embryos tested for a genetic disease and found to be unaffected do not develop into an ongoing pregnancy, likely owing to aneuploidy. It is now possible to combine PGD with PGS, enabling only unaffected euploid embryos to be selected.
Non-invasive embryo selection
Within the IVF lab, there is a huge focus on whether euploid embryos can be selected using non-invasive (not biopsying) techniques. Time-lapse monitoring involves culturing embryos in an undisturbed environment with frequent photography to aid the selection of embryos more likely to result in a healthy live birth.5study by Rubio el al suggested that ongoing pregnancy rates may be increased by nine per cent (51 per cent versus 42 per cent) using time-lapse technology, in part by decreasing miscarriage rates. Other non-invasive technologies, metabolomics and transcriptomics, involve analysis of the fluid surrounding the embryo or the cells nurturing the oocytes in order to gain more information about the embryo.
What does the future look like?
PGD and PGS are selection tools to enable the IVF clinician to choose the best embryo. There are many PGD and PGS cycles where there are no normal embryos available. There are also parents who do not wish to discard their affected embryos. The future may be about fixing the embryo. In a recent report from China, gene-editing techniques were attempted on human embryos with thalassaemia.6 We are still a long way from knowing whether this is possible, safe or useful.
In conclusion, PGD and PGS have become robust technologies that continue to improve. Nonetheless, most couples using assisted reproduction do not require or benefit from either PGD or PGS.
References
- Handyside AH, Kontogianni EH, Hardy K, Winston RM. Pregnancies from biopsied human preimplantation embryos sexed by Y-specific DNA amplication. Nature. 1990; 344 (6268):768-770.
- Handyside AH, Lesko JG, Tarin JJ, Winston RML. Birth of a normal girl after IVF and Pre implantation Diagnostic Testing for cystic fibrosis. NEJM. 1992; 327:905-909.
- Scott RT Jr, Upham KM, Forman EJ et al. Cleavage stage biopsy significantly impairs human embryic implantation potential while blastocyst biopsy does not: a randomised and paired clinical trial. Fertil Steril. 2013; 100(3): 624-630.
- Munne S, Weier HU, Stein J et al. A fast and efficient method for simultaneous X and Y in situ hybridization of human blastomeres. J Asst Reprod Genet. 1993; 10(1): 82-90.
- Rubio I, Galán A, Larreategui Z et al. Clinical Validation of Embryo Culture and Selection by Morphokinetic Analysis: A Randomized, Controlled Trial of the EmbryoScope. Fertil Steril. 2014;102:1287-1294.e5.
- Puping Liang, Yanwen Xu, Xiya Zhang et al. CRISPR/Cas9-mediated gene editing in human tripronuclear zygotes. Protein & Cell. May 2015; 6(5):363-372.






Nice site and good information and thanks for sharing this information site and articles good knowledge…